Icaritin nanoparticles as well as preparation method and application thereof
A technology of icariin and nanoparticles, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of low bioavailability, low biological potency, and limited applications, etc. problem, achieve the effect of improving water solubility and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of icariin nanoparticles
[0035] (1) citrus pectin (Shanghai McLean Biochemical Technology Co., Ltd.) is added to the 0.0375mol / L aqueous hydrochloric acid solution according to a material-to-liquid ratio of 10g / L, and after high-speed shear dispersion, 50 ° C is heated to be completely dissolved to obtain a pectin solution;
[0036] (2) under stirring condition, add icariin (Sichuan Vikki Biotechnology Co., Ltd.) to 0.15mol / L sodium hydroxide aqueous solution according to the material-to-liquid ratio of 10g / L, and obtain icariin solution after completely dissolving;
[0037] (3) under stirring conditions (500rpm / min), add the icariin solution of step (2) to the pectin solution of step (1) according to the volume ratio of 1:4, and continue stirring for 2h at room temperature, 10000g Centrifuge, and take the upper layer solution as Icaritin-pectin nanoparticles (IPNPs).
[0038] The described IPNPs were characterized as follows:
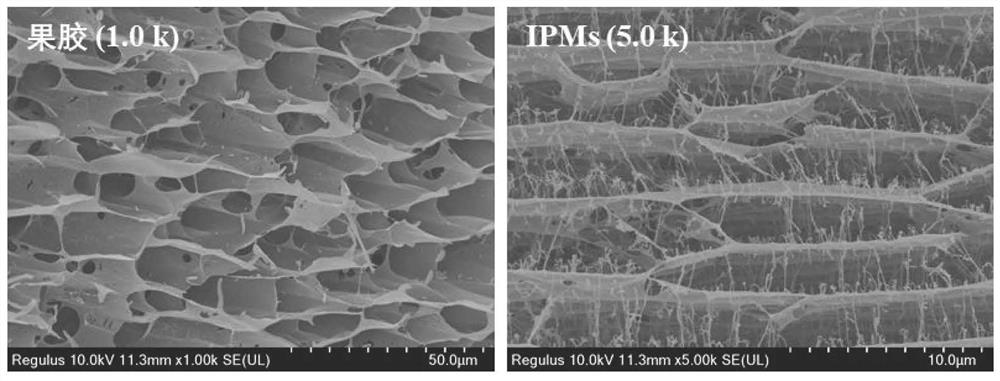
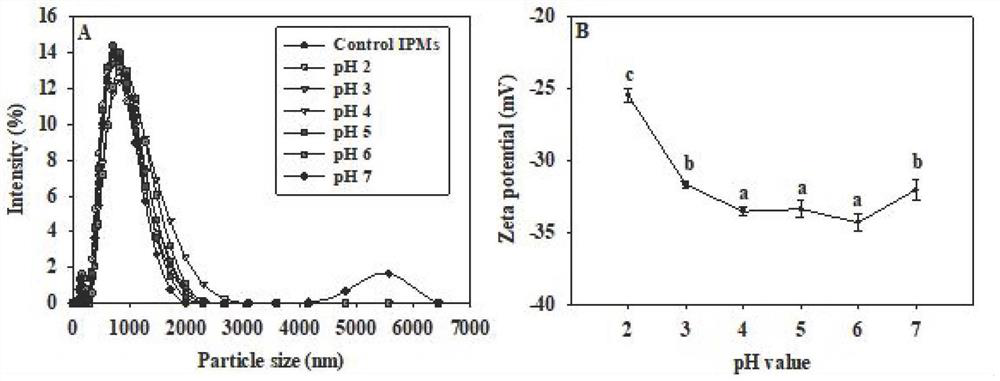
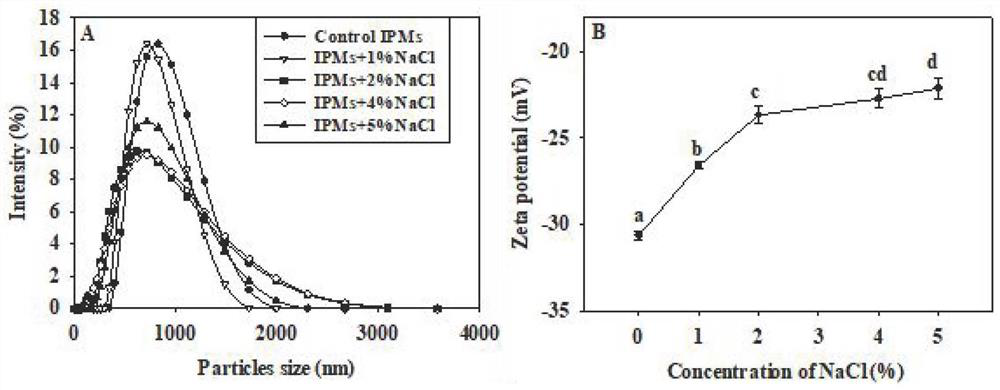
[0039] (1) Ana...
Embodiment 2
[0073] Example 2: Preparation of icariin nanoparticles
[0074] (1) citrus pectin (Shanghai McLean Biochemical Technology Co., Ltd.) is added to the 0.02mol / L hydrochloric acid aqueous solution according to a material-to-liquid ratio of 5g / L, after high-speed shearing and dispersing, heating at 45°C to complete dissolution to obtain a pectin solution;
[0075] (2) under stirring condition, add icariin (Sichuan Vikki Biotechnology Co., Ltd.) to 0.4mol / L aqueous sodium hydroxide solution according to the material-to-liquid ratio 50g / L, and obtain icariin solution after completely dissolving;
[0076] (3) under stirring condition (500rpm / min), add the icariin sodium hydroxide aqueous solution of step (2) to the pectin solution of step (1) according to the volume ratio of 1:20, and continue stirring at room temperature for 1.5 After h, centrifuge at 10000g, and take the upper layer solution as icariin nanoparticles (IPNPs).
[0077] The average drug loading of the IPNPs obtained ...
Embodiment 3
[0078] Example 3: Preparation of icariin nanoparticles
[0079] (1) citrus pectin (Shanghai McLean Biochemical Technology Co., Ltd.) is added to the 0.0125mol / L hydrochloric acid aqueous solution according to a material-to-liquid ratio of 10g / L, and after high-speed shear dispersion, 45 ° C is heated to be completely dissolved to obtain a pectin solution;
[0080] (2) under stirring condition, add icariin (Sichuan Vikki Biotechnology Co., Ltd.) to 0.06mol / L sodium hydroxide aqueous solution according to the material-to-liquid ratio 5g / L, and obtain icariin solution after completely dissolving;
[0081] (3) under stirring condition (500rpm / min), add the icariin sodium hydroxide aqueous solution of step (2) to the pectin solution of step (1) according to the volume ratio of 1:4, and continue stirring for 3h at room temperature After centrifugation at 10000g, the upper layer solution was taken as icariin nanoparticles (IPNPs). The average drug loading of the IPNPs obtained in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com