CLEC18A protein-based medicine for preventing and inhibiting inflammatory response of organism and application of CLEC18A protein-based medicine
A CLEC18A, inflammatory response technology, applied in the field of biomedicine, can solve problems such as unreported effects, achieve the effects of preventing and inhibiting inflammatory effects, reducing potential damage and side effects, and reducing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
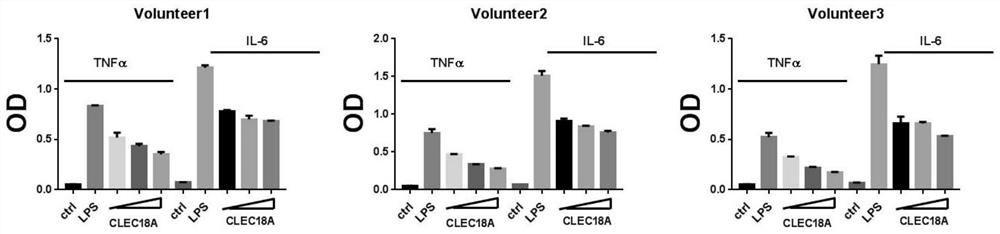
[0022] Recombinant human CLEC18A (CUSABIO, Cat. No. CSB-EP005521HU) can significantly reduce the production of inflammatory factors TNFα and IL-6 in human peripheral blood mononuclear cells induced by LPS, a cell wall component of E. coli.
[0023] Method and model establishment: Peripheral blood was drawn from healthy people, peripheral blood mononuclear cells were isolated, and plated in 24-well cell culture plates, 5 × 10 cells per well. 5 cell. Grouping: control group with medium, LPS group with 100ng / ml LPS, treatment group with 100ng / ml LPS first, 2 hours later, 20ng / ml, 50ng / ml and 100ng / ml recombinant human CLEC18A were added respectively. After 6 hours, the cell culture supernatant was taken, and the concentrations of TNFα and IL-6 were detected by ELISA. The result is as figure 1 shown, recombinant human CLEC18A was found to significantly attenuate LPS-induced production of TNFα and IL-6 in peripheral blood mononuclear cells.
Embodiment 2
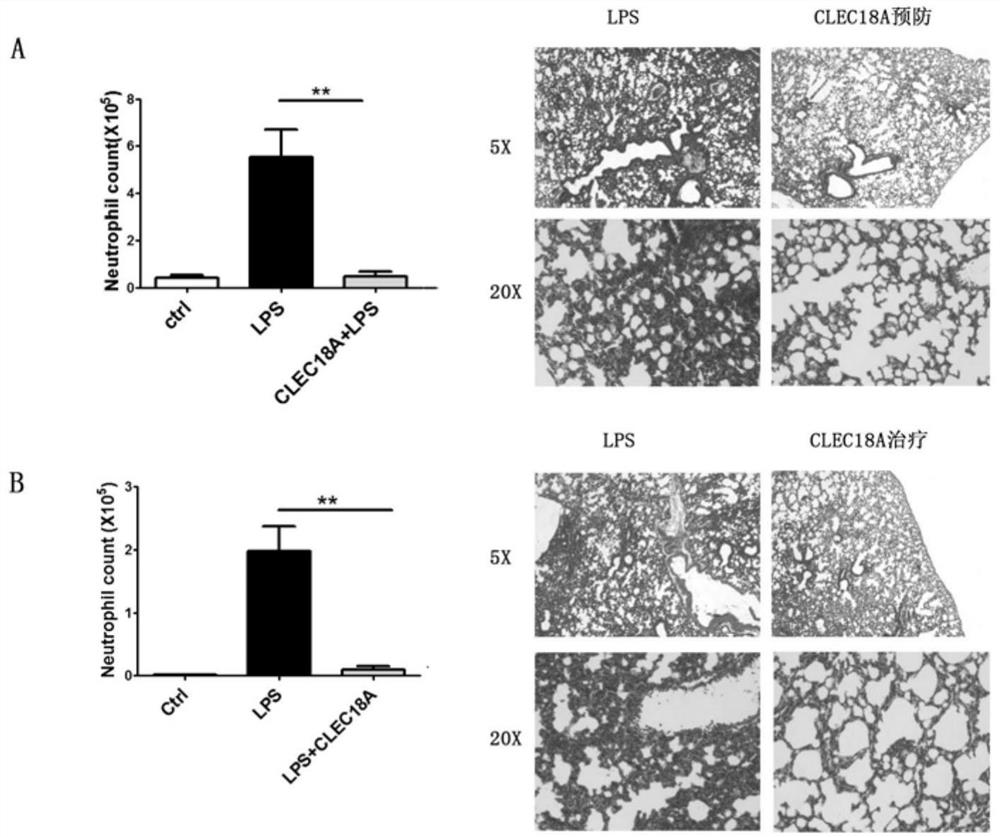
[0025] Treatment of LPS-induced acute lung injury in mice by recombinant mouse CLEC18A (CUSABIO, Cat. No. CSB-YP759740MO).
[0026] 8-10-week-old C57BL / 6 mice were randomly divided into 3 groups with 4 mice in each group. All mice were anesthetized by sevoflurane inhalation before the experiment.
[0027] Prevention experiment: mice in the control group were intubated with 100 μl PBS; LPS group was intubated with 100 μl LPS; mice in the prevention group were intraperitoneally injected with 1 ng / g of recombinant mouse CLEC18A for 2 hours and then intratracheally injected with LPS.
[0028] Treatment experiment: the control group and the LPS group are the same as above. 2 hours after intratracheal injection of LPS in the treatment group, 2ng / g recombinant mouse CLEC18A was intraperitoneally injected.
[0029] Results: After 6 hours, the mice were washed with bronchoalveolar lavage fluid (BALF), and CD11b in BALF was detected by flow cytometry. high Gr-1 high Neutrophils were ...
Embodiment 3
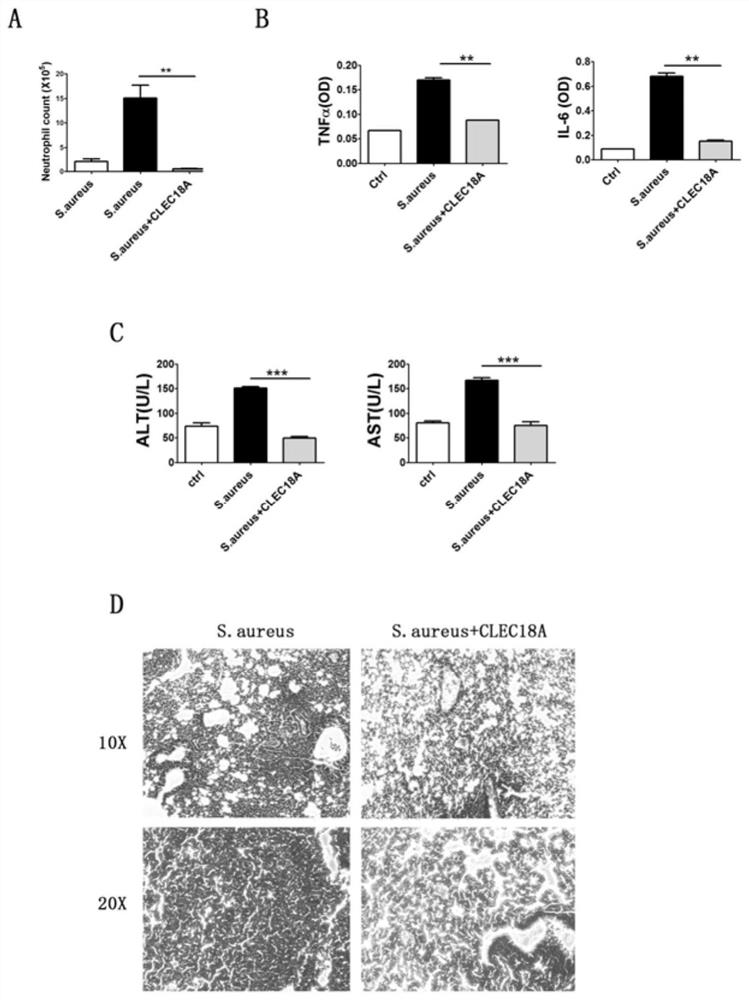
[0032] Treatment of S. aureus-induced pulmonary infection by recombinant mouse CLEC18A.
[0033]Methods and model establishment: C57BL / 6 mice aged 8-10 weeks were randomly divided into 3 groups with 4 mice in each group. All mice were subjected to sevoflurane inhalation anesthesia before experiments. Grouping: mice in the control group were slowly inhaled with 100 μl of PBS, and mice in the bacterial group were inhaled with 100 μl of S. aureus dissolved in PBS (5×10 7 ). The mice in the treatment group were intraperitoneally injected with 1 ng / g CLEC18A of recombinant mice 4 hours after S. aureus inhalation.
[0034] Judgment of results: BALF of mice was washed after 24 hours, and CD11b in BALF was detected by flow cytometry high Gr-1 high Neutrophils were counted and the concentrations of TNFα and IL-6 in BALF were detected by ELISA. Lung tissue for pathological HE staining. Alanine transaminase (ALT) and aspartate aminotransferase (AST) were detected in mouse serum to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com