Freeze-dried preparation of recombinant bacillus calmette guerin vaccine for treatment and preparation method and application of freeze-dried preparation
A technology for reconstituting BCG vaccine and freeze-dried preparations, which can be used in freeze-dried delivery, medical preparations of inactive ingredients, drug combinations, etc., can solve problems such as concentration increase, and achieve the effects of preventing damage, high quantity, and good clinical application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Preparation of the freeze-dried preparation of recombinant BCG for therapeutic use of the present invention
[0036] (1), the preparation of stabilizer: add the following mass fraction components to the water for injection: dextran 3.0%, sucrose 4.0%, sodium glutamate 1.0%, potassium chloride 1.0%, trehalose 4.0%, Tween 0.01%, then adjust the pH to 7.3-7.5 with 10% sodium hydroxide solution, and sterilize for 50 minutes at 116°C and 0.16MPa.
[0037] (2) Passaging of production strains
[0038] 1. Recovery of working seeds: The working seeds of recombinant BCG were inoculated with Roche medium and cultured at 38°C for 2-3 weeks.
[0039] 2. Passaging Sutong medium: Roche strains are inoculated in Sutong medium, and cultured at a suitable temperature (38° C.) and a suitable time (2-3 weeks).
[0040] (3) Preparation of stock solution
[0041] 1. Production of biofilm culture: use the production strain to pass the Sutong medium twice with an appropriate inoc...
Embodiment 2
[0052] Embodiment 2, the preparation of recombinant BCG freeze-dried preparation
[0053] Preparation of stabilizer: add the following components in water for injection: dextran 3.0%, sucrose 4.0%, sodium glutamate 2.0%, potassium chloride 1.0%, trehalose 4.0%, tween 0.01%, and then Adjust the pH to 7.3-7.5 with 10% sodium hydroxide solution, and sterilize for 50 minutes at 116° C. and 0.16 MPa.
[0054] The rest of the method steps are the same as in Example 1.
experiment example 1
[0064] Experimental example 1. Quality evaluation of the preparation of the present invention
[0065] 1. Experimental method
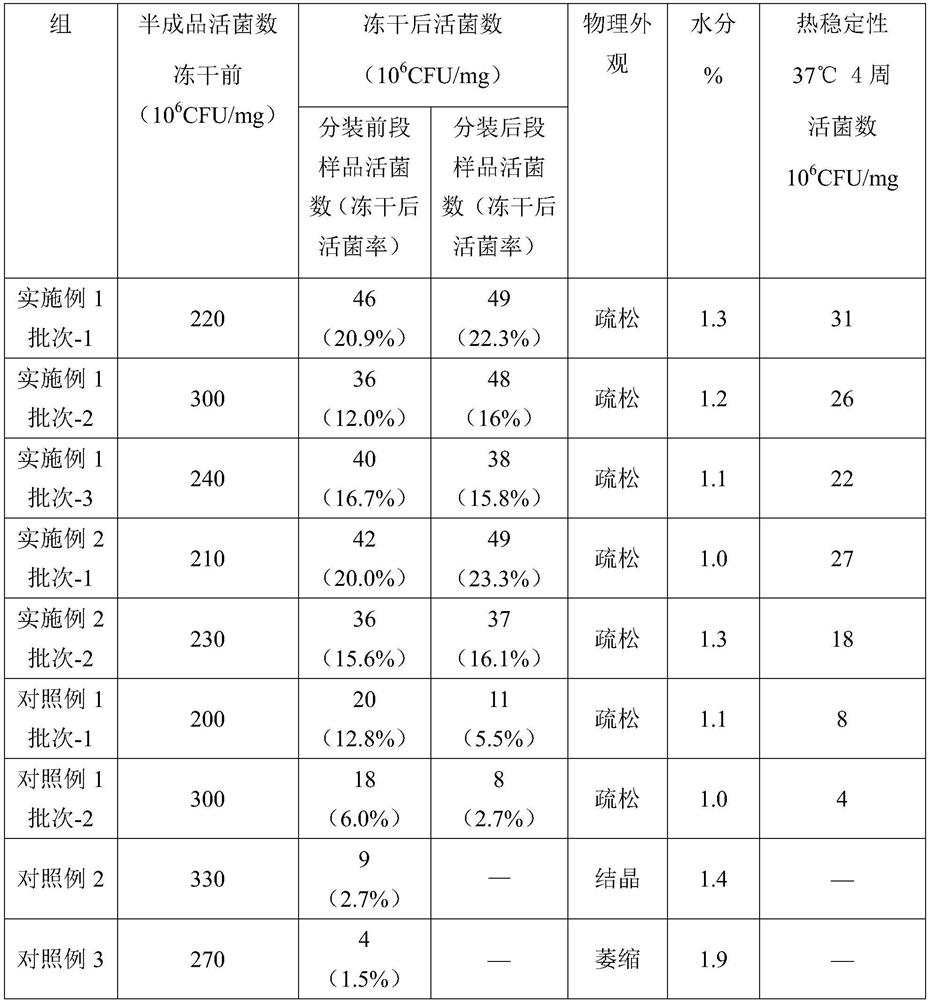
[0066] Performances such as the number of viable bacteria, physical appearance, moisture, and thermal stability before and after freeze-drying of the preparations of the Examples and Comparative Examples were evaluated and compared. The results are shown in Table 2.
[0067]
[0068] As can be seen from the results in the table, the reconstituted BCG freeze-dried preparations (Examples 1 and 2) with the stabilizer of the specific composition of the present invention are added. at 30×10 6 CFU / mg; the product has good thermal stability, and the viable cell rate is more than 50% compared with the storage condition at 4°C (that is, the viable cell count after freeze-drying); and it is conducive to the dispersion of recombinant BCG. The consistency of the number of viable bacteria in the latter sample is good, reflecting that the preparation of the pr...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap