A strain of Bifidobacterium bifidum and its application in relieving intestinal injury
A technology of Bifidobacterium bifidum and viable count, applied in the field of microorganisms to reduce mortality, alleviate or prevent acute colon tissue damage, and improve weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Isolation and screening of Bifidobacterium bifidum CCFM1172
[0035] 1. Sample Collection
[0036] The stool samples of healthy infants in Jiangning District, Nanjing City, Jiangsu Province were collected. The samples were placed in a preservation tube containing 30% glycerol, and stored in an incubator with an ice pack. After being brought back to the laboratory, they were quickly placed in a -80°C refrigerator for separation. filter.
[0037] 2. Isolation and purification of bifidobacteria
[0038] (1) Dilution coating: In a sterile environment, take about 0.5 g of a mixture of 30% glycerol and feces and add it to a 10 mL centrifuge tube of 4.5 mL of normal saline to obtain 10 -1 Diluent, repeat the above dilution steps to obtain 10 in turn -2 , 10 -3 , 10 -4 , 10 -5 , 10 -6 Diluent;
[0039] (2) Coating culture: Pipette 100 μL of the above 10 -4 , 10 -5 , 10 -6Three gradient sample dilutions were prepared in MRS fixed medium containing 0.1% cyst...
Embodiment 2
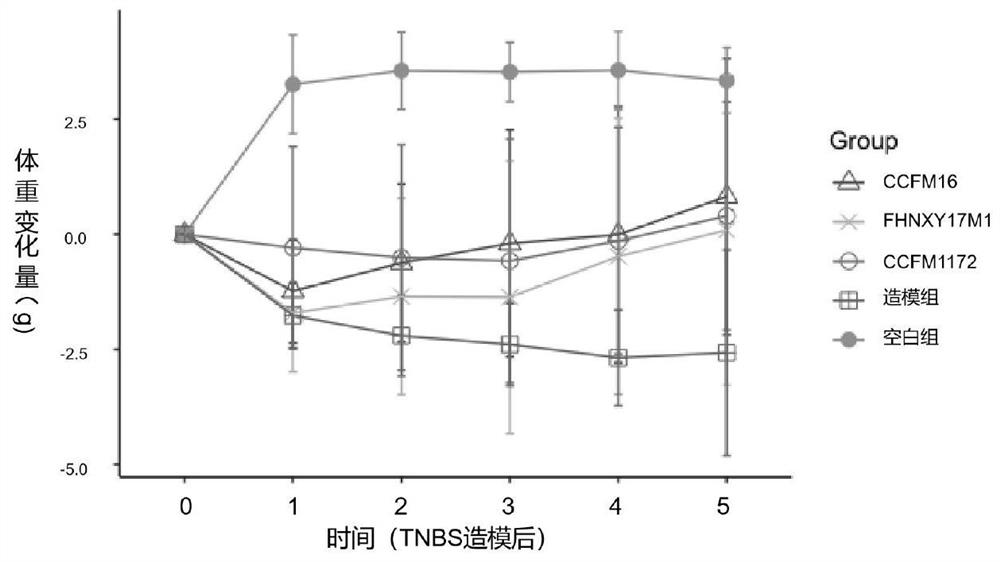
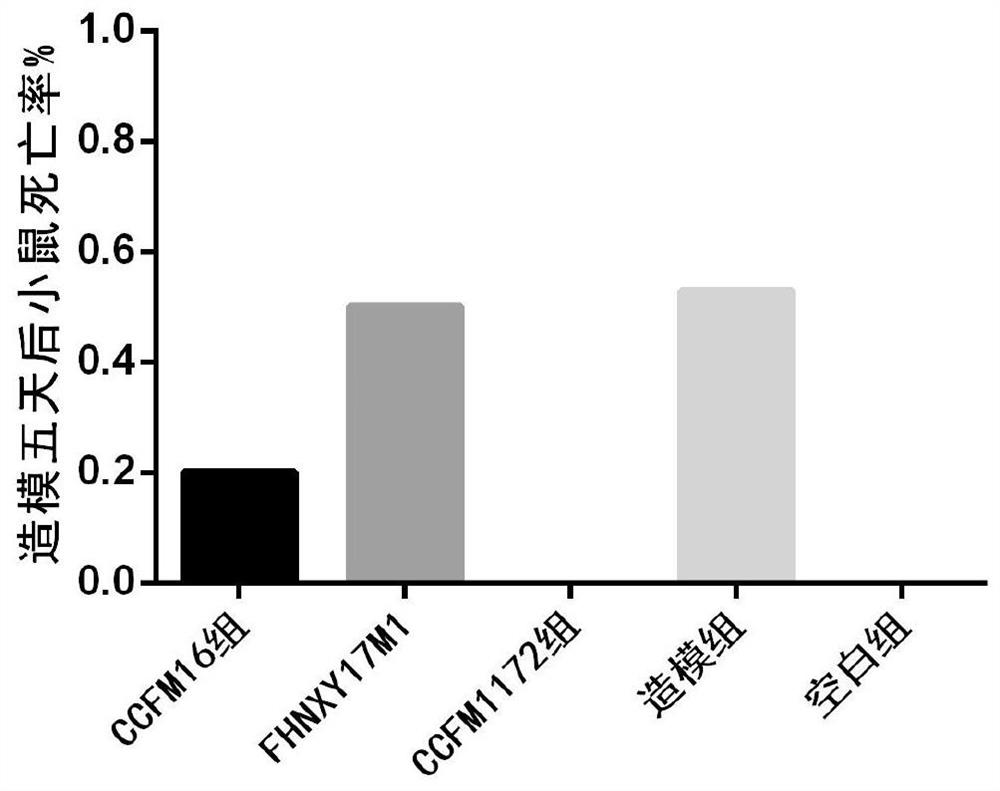
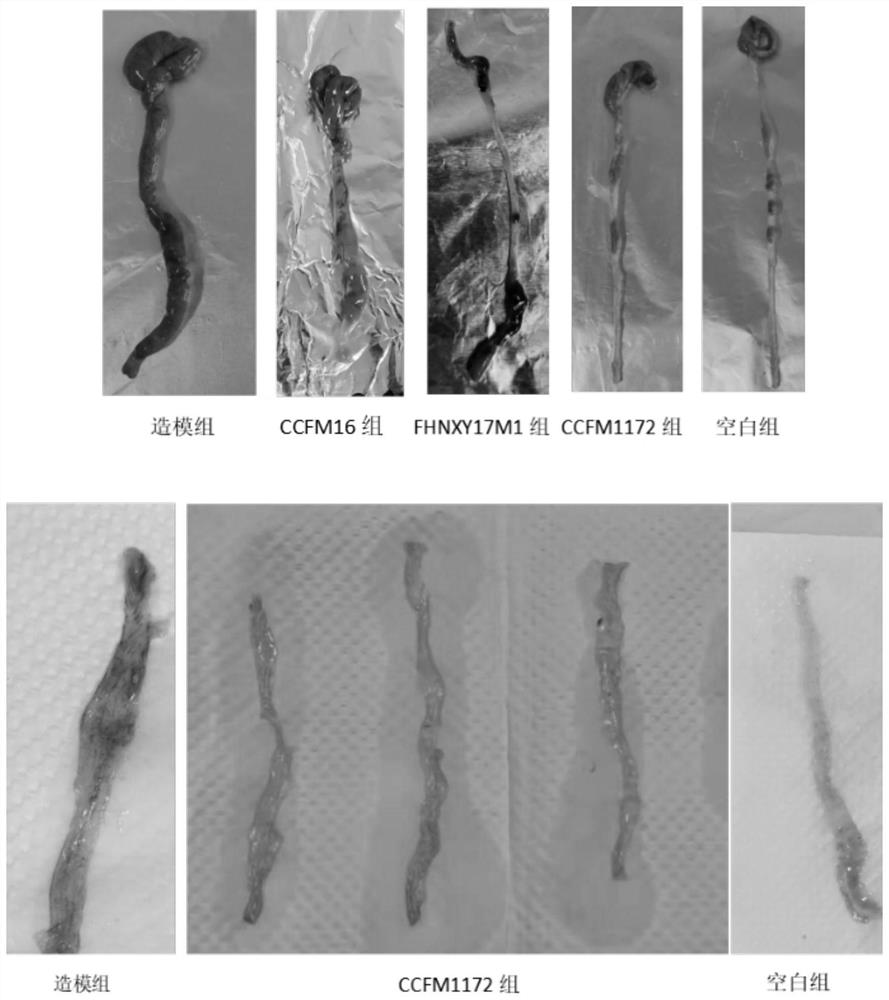
[0050] Example 2: Gavage of CCFM1172 before and after TNBS-induced colon injury has a significant protective effect
[0051] 1. Preparation of bacterial suspension
[0052] The Bifidobacterium bifidum CCFM1172 in the glycerol storage tube obtained in Example 1 was spread on the MRS solid plate, and cultivated in an anaerobic workstation at 37°C for 48 hours; a single colony was picked and inoculated in 5 mL MRS medium, and then placed in an anaerobic workstation. Incubate at 37 °C for 36 h; expand the activated bacterial solution to 1 L of MRS medium, cultivate in an anaerobic workstation at 37 °C for 2 days, centrifuge at 8000 r / min for 20 min, collect the bacterial slurry, and resuspend with PBS to adjust the viability. Bacteria count to 5×10 9 CFU / mL, aliquot into small portions, store in a 4°C refrigerator for later use, and use up within 5 days. Referring to the same method, Bifidobacterium bifidum CCFM16 and Bifidobacterium bifidum FHNXY17M1 bacterial suspensions scree...
Embodiment 3
[0068] Example 3: Therapeutic effect of CCFM1172 after TNBS-induced colon injury
[0069] 1. Experimental method
[0070] The preparation of the bacterial suspension and the experimental animals were the same as in Example 2.
[0071] Drug preparation method: 5% TNBS aqueous solution was prepared into 20ug / uL 35% ethanol aqueous solution with 75% ethanol solution and sterile water (ethanol concentration 35%, TNBS concentration 20ug / uL).
[0072] Eight-week-old male Balb / c mice were randomly divided into 3 groups, including a modeling group, a treatment group with Bifidobacterium bifidum CCFM1172, and a blank group, with 10 mice in each group. Mice were acclimated to the SPF barrier environment for one week and fasted for 24 hours. Then, the mice in the modeling group and the treatment group were rectally perfused with 50 uL of ethanolic TNBS solution using a syringe connected to a plastic hose, and the blank group was perfused with 35% ethanol solution in the same way. Ethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com