Aggregation-induced emission material as well as preparation method and application thereof
A technology of aggregation-induced luminescence and light wavelength, which is applied in the fields of wave energy or particle radiation treatment materials, pharmaceutical formulations, medical preparations with inactive ingredients, etc. In situ clearance, reducing drug resistance, and the effect of tight connection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
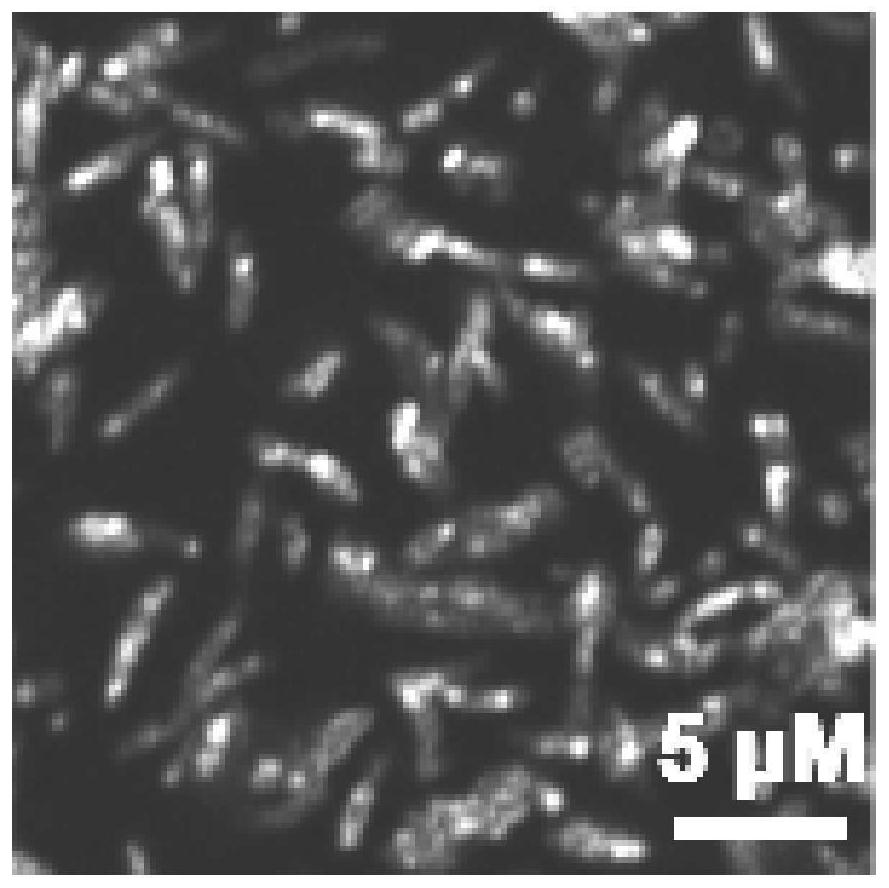
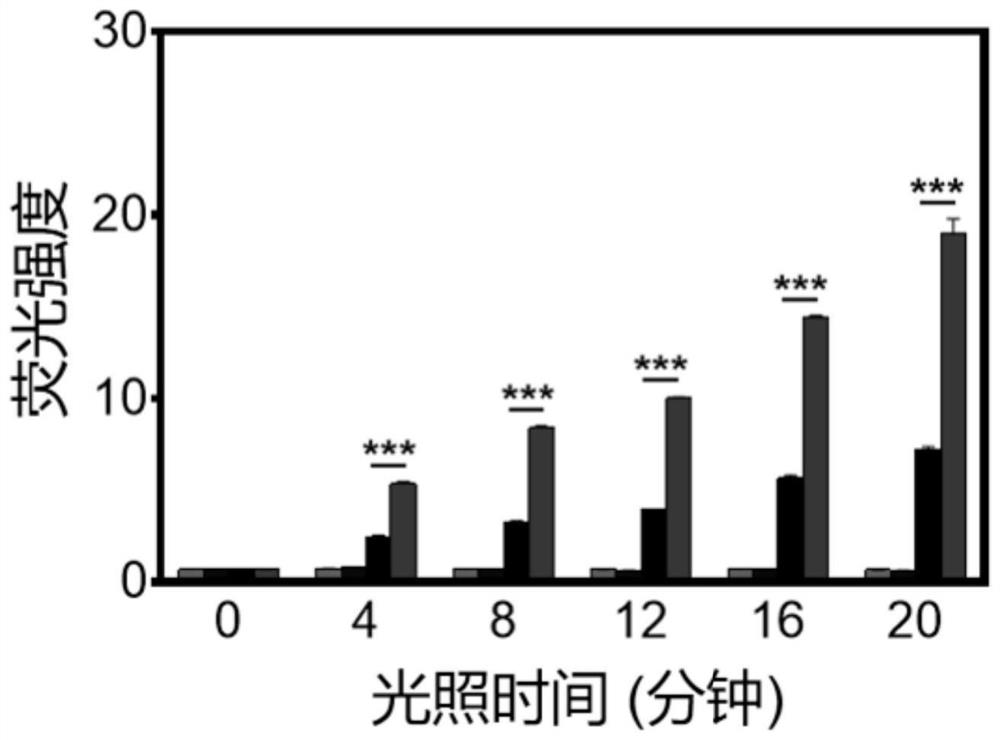
Image
Examples
preparation example Construction
[0036] A method for preparing an aggregation-induced luminescent material provided in the second aspect of the embodiments of the present application includes the following steps:
[0037] S01. provide a D-alanine derivative containing a first unsaturated bond and a 4-(2-(4-diphenylmethylaminophenylethene) pyridinium bromide derivative containing a second unsaturated bond;
[0038] S02. Combine the D-alanine derivative containing the first unsaturated bond and the 4-(2-(4-benzylaminophenylvinyl)pyridine bromide derivative containing the second unsaturated bond) Mixing treatment and click chemistry reaction are performed to obtain aggregation-induced luminescent materials.
[0039] The second aspect of the embodiments of the present application provides a method for preparing an aggregation-induced luminescent material. The preparation method is simple and easy to operate. The D-alanine derivative containing the first unsaturated bond and the D-alanine derivative containing the...
Embodiment A1
[0063] A kind of aggregation-induced luminescent material and preparation method thereof
[0064] The provided aggregation-induced light-emitting material is 3-tetrazine-D-alanine and 1-trans-cyclooctene-4-(2-(4-diphenylmethylaminophenylethene) pyridinium bromide) linked by chemical bonds.
[0065] The preparation method of aggregation-induced luminescent material comprises the following steps:
[0066] to provide 3-tetrazine-D-alanine and 1-trans-cyclooctene-4-(2-(4-benzylaminophenylethene)pyridinium bromide in a molar ratio of 1:0.1,
[0067] 3-Tetrazine-D-alanine and 1-trans-cyclooctene-4-(2-(4-dibenzylaminophenylethene)pyridine bromide were mixed and processed, stirred and mixed at room temperature and A click chemistry reaction is performed to obtain an aggregation-induced luminescent material.
Embodiment A2
[0069] A kind of aggregation-induced luminescent material and preparation method thereof
[0070] The provided aggregation-induced light-emitting material is 3-tetrazine-D-alanine and 1-trans-cyclooctene-4-(2-(4-diphenylmethylaminophenylethene) pyridinium bromide) linked by chemical bonds.
[0071] The preparation method of aggregation-induced luminescent material comprises the following steps:
[0072] to provide 3-tetrazine-D-alanine and 1-trans-cyclooctene-4-(2-(4-benzylaminophenylethene)pyridinium bromide in a molar ratio of 1:1.5,
[0073] 3-Tetrazine-D-alanine and 1-trans-cyclooctene-4-(2-(4-dibenzylaminophenylethene)pyridine bromide were mixed and processed, stirred and mixed at room temperature and A click chemistry reaction is performed to obtain an aggregation-induced luminescent material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Laser wavelength | aaaaa | aaaaa |
| Laser intensity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com