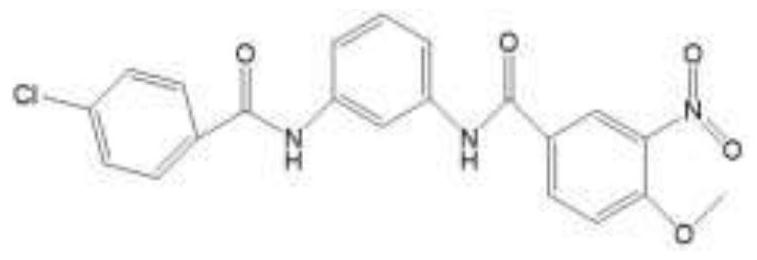
N-(3-(3-chlorobenzamide) phenyl)-4-methoxy-3-nitrobenzamide and preparation method and application thereof
A technology of nitrobenzamide and chlorobenzamide, which is applied in the field of compound synthesis, can solve the problem of lack of high-efficiency and specific clinical treatment drugs for colon cancer, achieve inhibition of proliferation and growth, simple preparation method, and easy-to-obtain preparation raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This example describes N-(3-(3-chlorobenzamide)phenyl)-4methoxy-3-nitrobenzamide and its preparation method. The N-(3-(3-chlorobenzamide) The structural formula of carboxamide) phenyl)-4-methoxy-3-nitrobenzamide is as follows figure 1 As shown, the preparation method of this N-(3-(3-chlorobenzamide) phenyl)-4 methoxyl group-3-nitrobenzamide is as follows:
[0043]
[0044] Wherein, the molar ratio of m-phenylenediamine, triethylamine and m-chlorobenzoyl chloride is 1:2:1, 3-nitro-4-methoxybenzoic acid, N,N-diisopropylethylamine, The molar ratio of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride was 1:2:2.
[0045] Specifically, the preparation method of this N-(3-(3-chlorobenzamide)phenyl)-4methoxy-3-nitrobenzamide is illustrated below:
[0046] Dissolve 2.16 g m-phenylenediamine (20 mmol) in 100 mL of dichloromethane, add 5.5 mL of triethylamine under an ice bath to obtain a mixed solution 1, and dissolve 3.5 g of m-chlorobenzoyl chloride (20 mmol) in 20...
Embodiment 2
[0052] Example 2 Related Cell Tests
[0053] (1) Cell culture
[0054] HCT116, HT29, SW620, HEK293T and FHC used in this example were purchased from the American Type Culture Collection (ATCC, Virginia, USA). HCT116 was cultured in McCoy's 5A medium (SH30200.01, Hyclone), and HT29, SW620, HEK293T and FHC were cultured in high-glucose DMEM medium (SH30022.01, Hyclone) at 37°C, Contains 5% CO 2 in a humidified incubator and supplemented with 10% fetal bovine serum (FBS, 10100147, Gibco) and 1% penicillin-streptomycin.
[0055] (2) Plasmid transfection and lentivirus infection
[0056] Plasmid constructs expressing T7-SKP2 and Myc-SKP2 were obtained from Shandong Weizhen Biotechnology Co., Ltd. Use Lipo8000 according to manufacturer's protocol TM Transfection reagents (Beyotime) were used to transfect cells with the indicated plasmids. For stable transfection, use Lipo8000 TM Transfection Reagent Co-transfection of targeting plasmids, Pspax2 and pMD2G vectors into HEK293T ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com