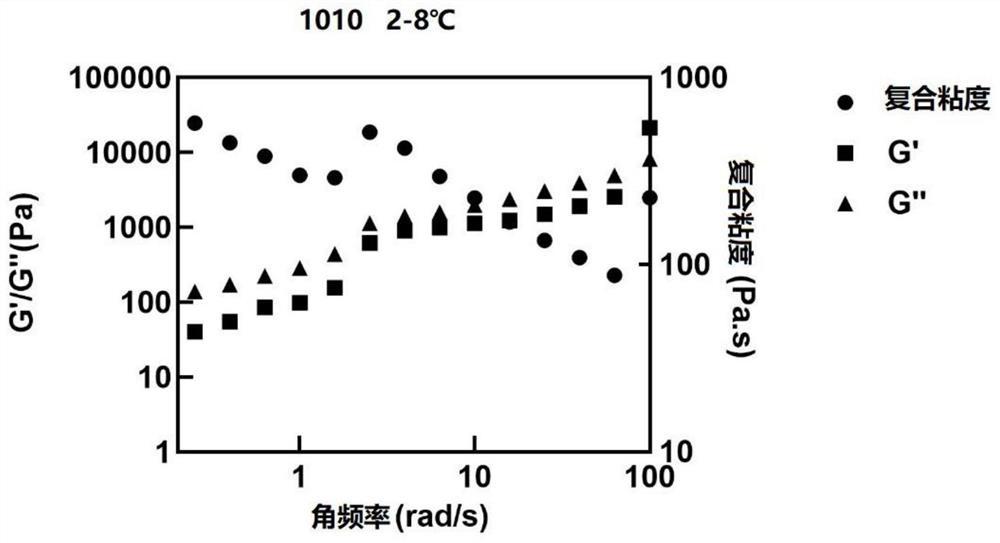
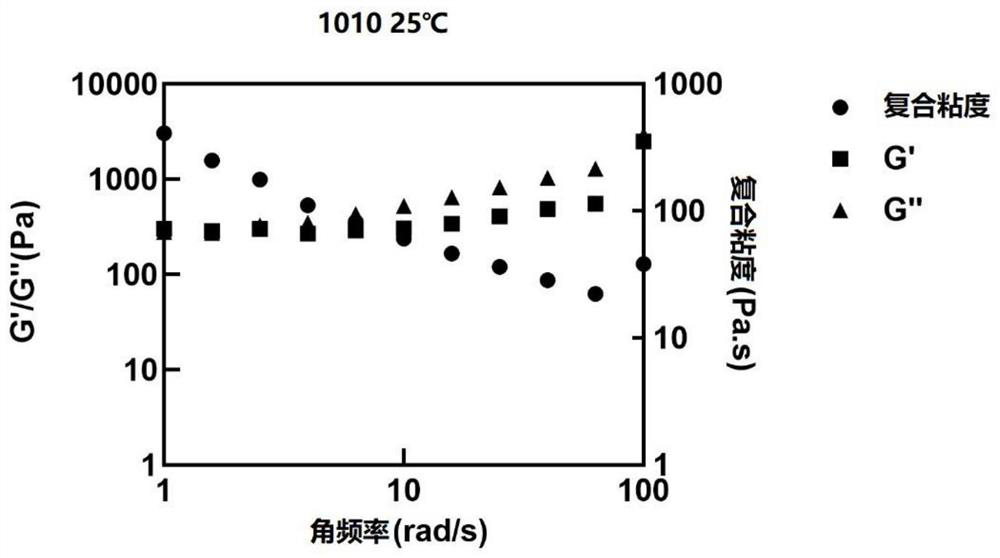
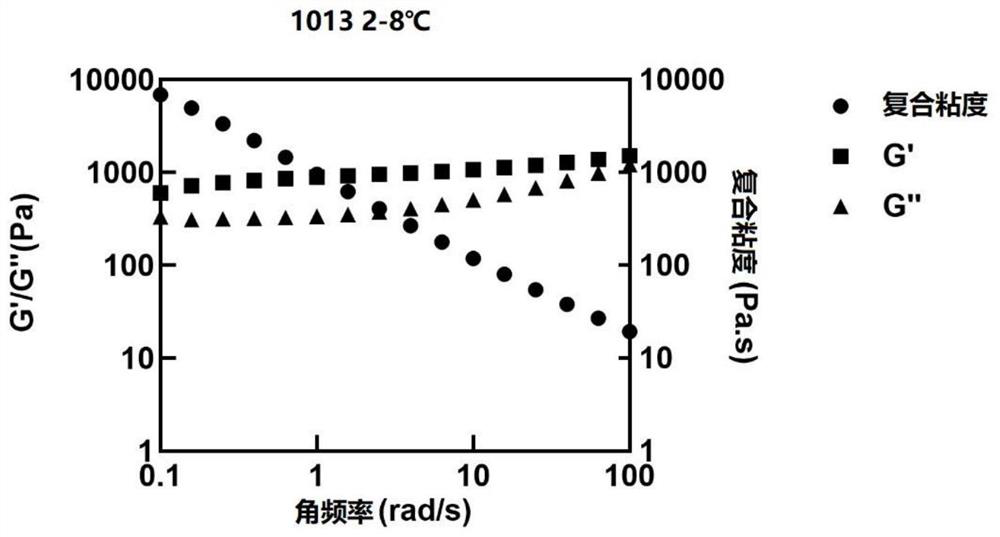
Sustained release preparation composition
A composition and compound technology, applied in the directions of drug combinations, anesthetics, anti-inflammatory agents, etc., can solve the problems of oil separation risk and oxidation, and achieve the effects of easy administration, improved oil retention, and improved discoloration problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Effect of Stabilizers on Appearance of Compositions
[0129] Prepare compositions containing different gelling factors as shown in Table 1-1 to Table 1-3, and place them at room temperature for a long time for 20 months. The change in appearance color of the composition was examined.
[0130] Table 1-1 Appearance color of the composition with glycerol monostearate as gelling factor after long-term storage
[0131]
[0132] Table 1-2 Appearance color of compositions with stearin as gelling factor after long-term storage
[0133]
[0134] Table 1-3 Appearance color of compositions with glyceryl behenate as gelling factor after long-term storage
[0135]
[0136] The present invention unexpectedly finds that the color of the oil-gel preparation containing only fatty acid glycerides as a gelling factor becomes darker to varying degrees during the long-term sample retention process, and the composition adding a stabilizer (such as SPC, HSPC) is placed for a long t...
Embodiment 2
[0138] Oil retention study of the composition
[0139] Pharmaceutical compositions containing different ratios of gelling factors were prepared according to each ingredient shown in Tables 2-1 to 2-9 below. 0.5 g of the pharmaceutical composition was weighed, placed in a centrifuge tube, centrifuged at different speeds, and the centrifuge tube was inverted after centrifugation to observe the oil separation of the composition.
[0140] Table 2-1 Study on oil retention of oil gel compositions without stabilizers
[0141]
[0142] Table 2-2 Comparison of oil retention of oil-gel compositions after adding stabilizer
[0143]
[0144] Table 2-3 Study on the oil retention of the oil-gel composition by the amount of stabilizer
[0145]
[0146] Table 2-4 Study on the oil retention of the oil gel composition by the amount of stabilizer
[0147]
[0148]
[0149] Table 2-5 Study on the oil retention of the oil gel composition by the amount of stabilizer
[0150]
...
Embodiment 3
[0161] Viscosity measurement of oil-gel compositions
[0162] Pharmaceutical compositions containing different amounts of stabilizers and organic solvents were prepared according to Tables 3-1 to 3-3. Mix the raw and auxiliary materials in the table at 70°C, stir while heating until a transparent and homogeneous solution is formed, and cool to room temperature to form a solid gel-like substance. Subsequently, the viscosity of the pharmaceutical composition was measured by a shaft method using a viscometer equipped with a No. 14 rotor, the detection temperature was 30° C. and the rotation speed was 10 rpm. The viscosity detection results are shown in Tables 3-1 to 3-3 below.
[0163] Table 3-1 Viscosity results of pharmaceutical compositions without stabilizers
[0164]
[0165] Table 3-2 Viscosity results of pharmaceutical compositions containing different stabilizers
[0166]
[0167] 3-3 Viscosity results of pharmaceutical compositions containing different amounts of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com