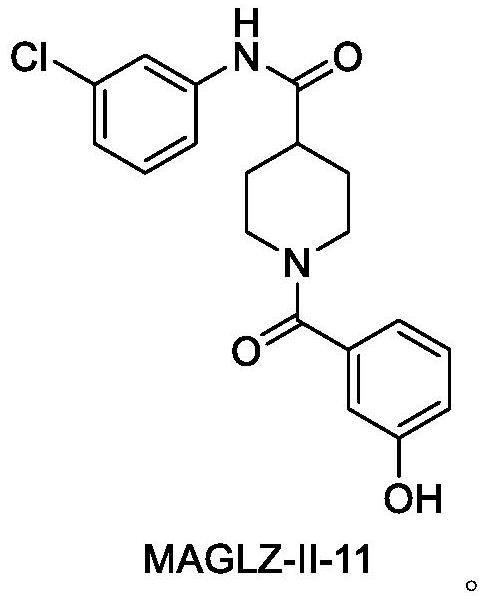
Medical application of MAGL inhibitor
A technology of MAGLII-11, 1.MAGLII-11, which is applied in the field of preparation of drugs for the prevention or treatment of alcoholic fatty liver disease, and can solve problems such as high price and damage to liver function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Effect of compound MAGLⅡ-11 on alcoholic fatty liver
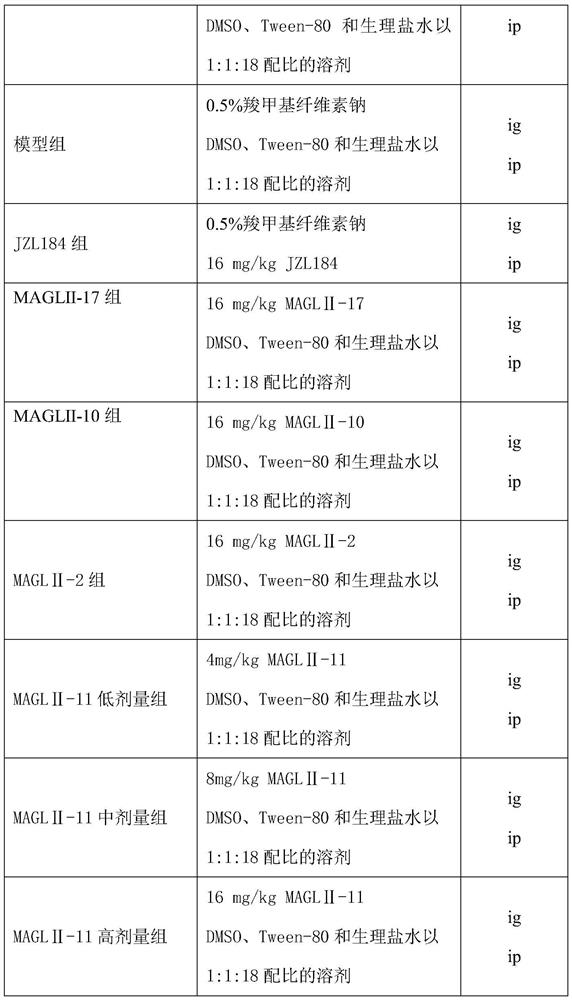
[0035] SD rats, 72, weighing 180-220g, half male and half male, were randomly divided into 9 groups, namely control group, model group, JZL184 group, MAGLⅡ-2 group, MAGLⅡ-10 group, MAGLⅡ-17 group, MAGLⅡ-11 group Low dose group, MAGLⅡ-11 medium dose group, MAGLⅡ-11 high dose group.
[0036] The control group was given normal saline by gavage and fed with normal feed; the rest of the rats were given free access to 10% alcohol and fed high-nutrient feed (normal feed supplemented with 10% whole milk powder, 10% egg yolk powder, 10% refined lard, etc.) after 6 weeks , divided into model group and each administration group, continued to feed high-nutrition feed and drink 10% alcohol for 4 weeks, and at the same time began to give drugs or distilled water by gavage for 4 weeks. 24 hours after the last administration, the orbits were bled, the serum was separated, and the serum liver function was measured; the an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com