Preparation method and application of porcine delta coronavirus inactivated vaccine
A coronavirus and inactivated vaccine technology, applied in the field of preparation of pig delta coronavirus inactivated vaccine, can solve the problems of high labor demand, high production cost, difficult to enlarge, etc., and achieves good safety, low production cost and easy effect of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Step-by-step scale-up culture of ST whole suspension cells
[0028] 1.1 ST full suspension cell recovery: Take out the ST full suspension cell cryovial from the liquid nitrogen tank, immediately put it in a 37°C water bath, and shake the cryovial gently to thaw the liquid as soon as possible. The cell suspension was centrifuged at 1000 r / min for 5 minutes. Discard the supernatant, use 20ml serum-free medium (CD ST 258, purchased from Gansu Jianshun Biotechnology Co., Ltd.) for each cryovial to suspend the cells in a 125ml shake flask, set at 37°C, 130r / min, containing 5% CO. 2 Shaker culture; daily cell counts ( figure 1 ), when the cell density reaches 4~6×10 6 When pcs / ml, the seeding density is 1×10 6 Numbers / ml were subcultured and expanded into 500ml shake flasks, about 100ml.
[0029] 1.2 Amplification of ST full suspension cells: place a 500ml shake flask at 37°C for 130r / min, containing 5% CO 2 Shaker culture; count cells every day, when the cell...
Embodiment 2
[0030] Example 2: Screening of porcine deltacoronavirus ST full suspension cell culture conditions
[0031] 2.1 To explore the density of inoculated cells, inoculated dose and cell density during inoculation
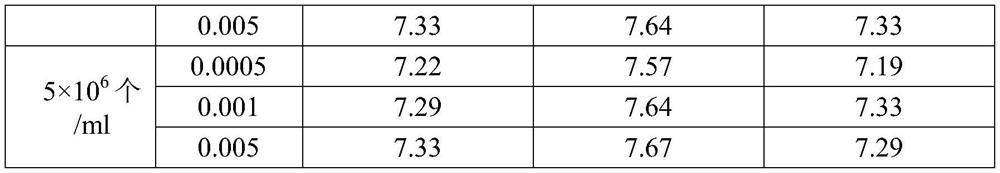
[0032] Test method: when the cells grow to 6 × 10 6 When cells / ml or more, adjust the cell density to 3 × 10 in serum-free medium containing trypsin 6 pcs / ml, 4×10 6 pcs / ml, 5.0×10 6cells / ml for three cell densities, and the final concentration of trypsin after adjustment was 40 μg / ml. According to MOI of 0.0005, 0.001, 0.005, access to pig delta coronavirus (from Jiangsu Academy of Agricultural Sciences, GenBank: KU665558.1), 37 ℃ 130r / min containing 5% CO 2 Shaker culture. Samples were taken every 6 hours for cell counting, and the cell density was 1 to 1.5 × 10. 6 pcs / ml, 0.5~1×10 6 pcs / ml and 0.1~0.5×10 6 Samples / ml were sampled for virus content determination and sterility test, and the best technological conditions for virus culture were screened.
[0033]...
Embodiment 3
[0044] Example 3: Preparation of pig deltacoronavirus inactivated vaccine
[0045] 3.1 Production of porcine delta coronavirus culture solution: according to the method of Example 1, the ST full suspension cells were gradually enlarged into a 200L bioreactor for fermentation culture, and the culture volume was 100L. When the cells grew to 6 × 10 6 When the number / ml or more, the pig delta coronavirus was inserted according to the optimal process determined in Example 2. Specifically: when the cell density is 6 × 10 6 1 / ml, supplement with 6L trypsin (original concentration is 1mg / ml), 44L serum-free medium, 19ml porcine delta coronavirus (original titer is 10 7.50 TCID 50 / ml), continue the fermentation culture, and control the parameters of the reactor as follows: dissolved oxygen 40-50%, pH 7.0-7.2, stirring 80-100 rpm; sampling every 6 hours for cell counting, when the cell density drops to 0.5-1.0 × 10 6 Cell cultures were harvested at cells / ml.
[0046] 3.2 Removal of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com