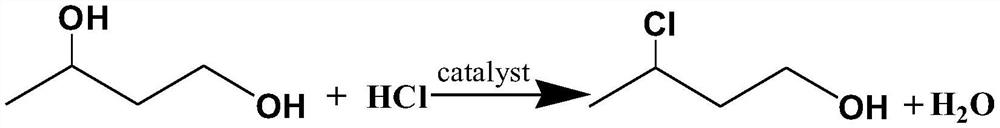
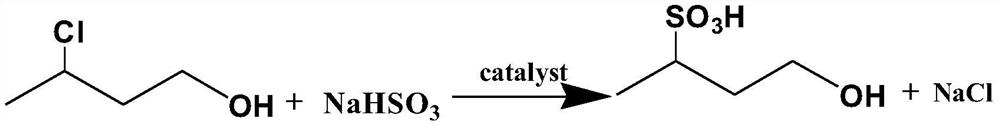
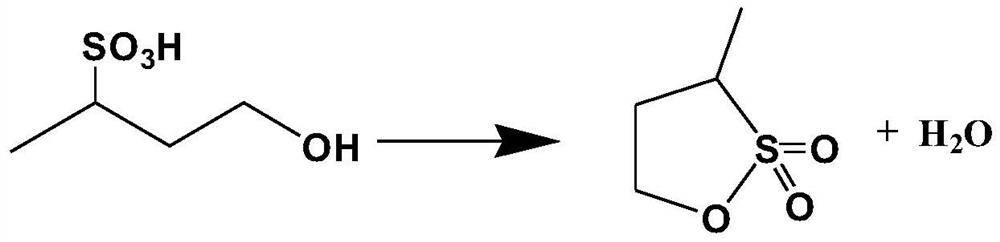
Method for preparing 2, 4-butane sultone
A technology of butane sultone and hydroxybutane sulfonic acid, which is applied in the field of synthesis of fine chemical intermediates, can solve the problems of inconvenient industrial continuous production, harsh reaction conditions, complex reaction steps, etc. Mild and stable conditions, the effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of 2,4-butane sultone, comprises the steps:
[0043] A. Put 1,3-butanediol and 27.5wt% hydrochloric acid into the reaction vessel and mix thoroughly, add catalyst strong basic resin (the amount of strong basic resin is 2.5% of the mass of 1,3-butanediol), and start heating And stir, slowly introduce hydrogen chloride gas, the molar ratio of 1,3-butanediol and HCl (the total amount of HCl in hydrochloric acid and the total amount of HCl introduced) is 1:1.1, and the temperature rises and refluxes to generate a nucleophilic substitution reaction, and the reaction temperature is controlled at 55 ° C The reaction was carried out for 9 hours to obtain an oily crude product containing 3-chlorobutanol; the 3-chlorobutanol crude product was rectified under reduced pressure, and fractions at 73-74° C. (16 mmHg) were collected;
[0044]B, in A, 3-chlorobutanol and sodium sulfite aqueous solution (sodium sulfite mass fraction is 25wt%) are dropped into the r...
Embodiment 2
[0049] The preparation method of 2,4-butane sultone, comprises the steps:
[0050] A. Put 1,3-butanediol and 30.0wt% hydrochloric acid into the reaction vessel and mix thoroughly, add catalyst strong basic resin (the amount of strong basic resin is 3.5% of the mass of 1,3-butanediol), and start heating And stir, slowly introduce hydrogen chloride gas, the molar ratio of 1,3-butanediol and HCl (the total amount of HCl in hydrochloric acid and the total amount of HCl introduced) is 1:1.3, and the temperature rises and refluxes to generate a nucleophilic substitution reaction, and the reaction temperature is controlled at 70 ° C The reaction was carried out for 7 hours to obtain an oily crude product containing 3-chlorobutanol; the 3-chlorobutanol crude product was rectified under reduced pressure, and fractions at 73-74° C. (16 mmHg) were collected;
[0051] B, in A, 3-chlorobutanol and sodium sulfite aqueous solution (sodium sulfite mass fraction is 30wt%) are dropped into reac...
Embodiment 3
[0056] The preparation method of 2,4-butane sultone, comprises the steps:
[0057] A. Put 1,3-butanediol and 32.5wt% hydrochloric acid into the reaction vessel and mix thoroughly, add catalyst strong basic resin (the amount of strong basic resin is 4.5% of the mass of 1,3-butanediol), and start heating And stir, slowly introduce hydrogen chloride gas, the molar ratio of 1,3-butanediol and HCl (the total amount of HCl in hydrochloric acid and the total amount of HCl introduced) is 1:1.5, and the temperature rises and refluxes to generate a nucleophilic substitution reaction, and the reaction temperature is controlled to 85 ° C The reaction was carried out for 5 hours to obtain an oily crude product containing 3-chlorobutanol; the 3-chlorobutanol crude product was rectified under reduced pressure, and fractions at 73-74° C. (16 mmHg) were collected;
[0058] B, 3-chlorobutanol and sodium sulfite aqueous solution (sodium sulfite mass fraction is 35wt%) in A are dropped into react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com