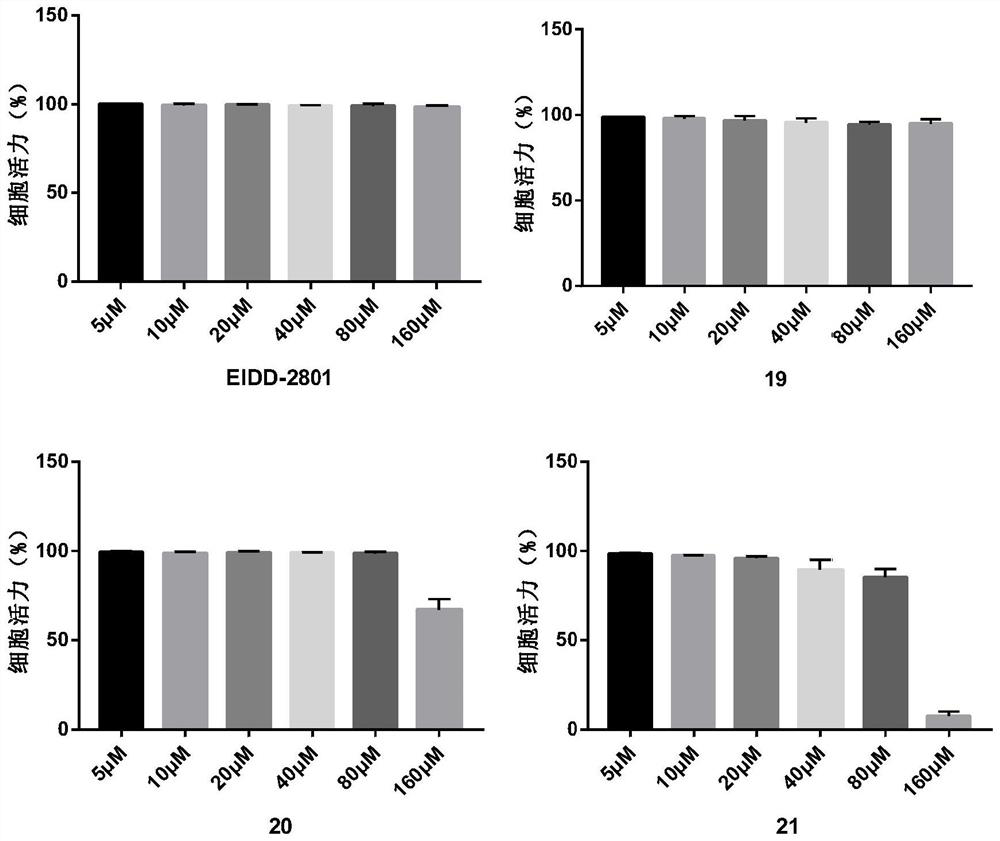
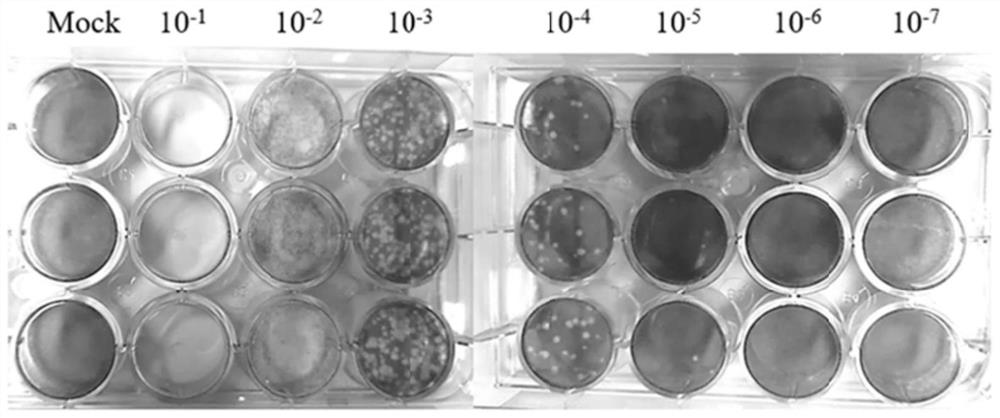
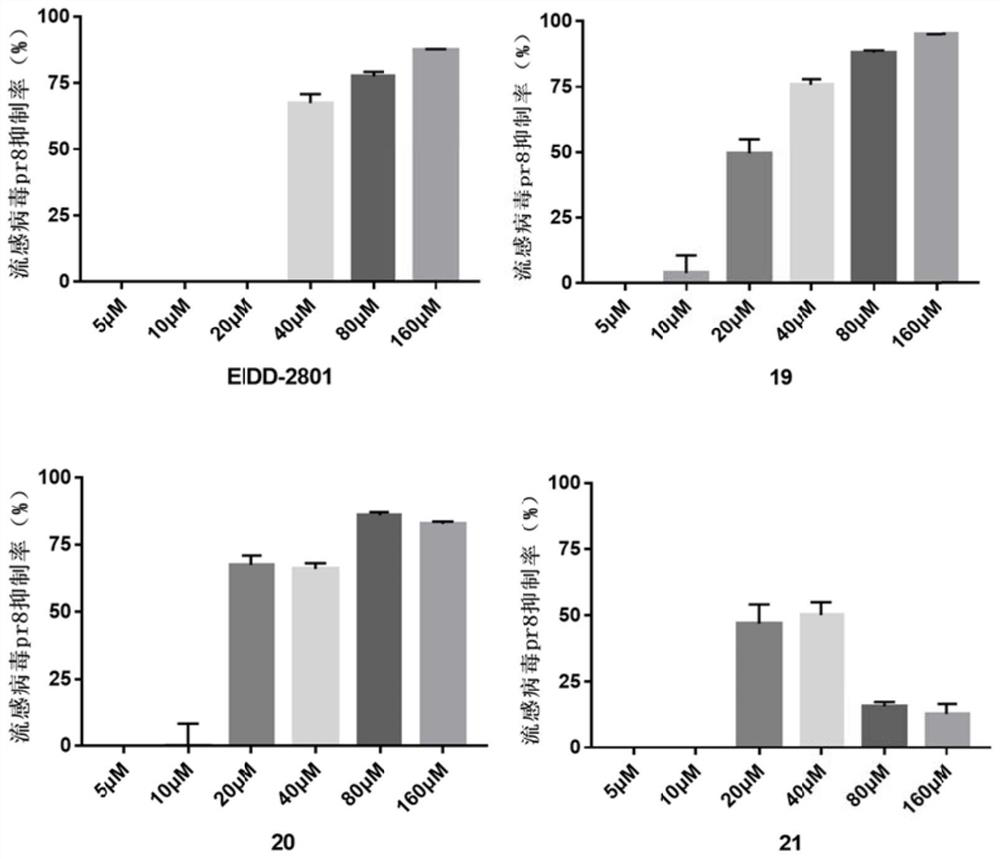
Fatty acid prodrug of nucleoside-like broad-spectrum antiviral drug as well as preparation method and application of fatty acid prodrug
A long-chain fatty acid, prodrug technology, applied in antiviral agents, preparation of sugar derivatives, chemical instruments and methods, etc., can solve problems such as poor oral bioavailability, improve metabolic stability, and improve biofilm permeability The effect of stability and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of Lauroyl Prodrug (Compound 19)
[0045]
[0046] In a 100ml reaction flask, N4-hydroxycytidine isopropyl ester (2.64g, 8mmol) was added, dichloromethane (30ml), triethylamine (3.36ml, 24mmol) were added, and lauroyl chloride was added at low temperature (0-10°C). (1.67ml, 7.2mmol) was slowly added dropwise to the reaction system, and then the reaction was naturally heated for 2-24h. After the reaction was completed, water was added to quench, and the organic phase was washed with saturated aqueous ammonium chloride solution, dried over anhydrous sodium sulfate, filtered, and concentrated. The obtained residue was purified by column chromatography (DCM:MeOH(v / v)=50:1) to obtain solid product. Yield 57%.
[0047] 1 H NMR (600MHz, chloroform-d) δ9.02(s, 1H), 7.08(d, J=8.3Hz, 1H), 5.87– 5.70 (m, 2H), 4.36–4.17 (m, 5H), 2.58 ( p, J=7.0Hz, 1H), 2.48 (t, J=7.6Hz, 2H), 1.69 (q, J=7.5Hz, 2H), 1.24-1.29 (m, 16H), 1.18 (d, J=7.0 Hz,6H),0.88(t, J=7....
Embodiment 2
[0050] Example 2: Preparation of Myristoyl Prodrug (Compound 20)
[0051]
[0052] Compound 20 was prepared as in Example 1 except that lauroyl chloride was replaced with myristoyl chloride. Finally a solid product is obtained. Yield 52%. 1 H NMR (600MHz, chloroform-d) δ9.02(s, 1H), 7.08(d, J=8.3Hz, 1H), 5.87-5.70(m, 2H), 4.36-4.17(m, 5H), 2.58( p, J=7.0Hz, 1H), 2.48 (t, J=7.6Hz, 2H), 1.69 (q, J=7.5Hz, 2H), 1.24-1.29 (m, 20H), 1.18 (d, J=7.0 Hz,6H),0.88(t,J=7.0Hz,3H).MS(ESI):m / z=540.30(M+H) + . 1 H NMR spectrum as Figure 9 shown.
Embodiment 3
[0053] Example 3: Preparation of Palmitoyl Prodrug (Compound 21)
[0054]
[0055] Compound 21 was prepared in the same manner as in Example 1, except that lauroyl chloride was replaced by palmitoyl chloride. Finally a solid product is obtained. Yield 55%.
[0056] 1 H NMR (600MHz, chloroform-d) δ9.02 (s, 1H), 7.08 (d, J=8.3 Hz, 1H), 5.87–5.70 (m, 2H), 4.36–4.17 (m, 5H), 2.58 ( p, J=7.0Hz, 1H), 2.48 (t, J=7.6Hz, 2H), 1.69 (q, J=7.5Hz, 2H), 1.24-1.29 (m, 24H), 1.18 (d, J=7.0 Hz,6H),0.88(t,J=7.0Hz,3H).MS(ESI):m / z=568.34(M+H) + . 1 H NMR spectrum as Figure 10 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dilution degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com