Single-domain antibody HCV-E2 of hepatitis C virus E2 protein and application of single-domain antibody HCV-E2
A technology of HCV-E2 and hepatitis C virus, which is applied in the field of biomedicine or biotechnology, can solve the problem of lack of single-domain antibodies to hepatitis C virus, etc., and achieve the effect of less cross-reaction, broad application prospects and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Immunopanning process of native single domain antibody against E2 protein
[0037] (1) Amplify the established natural single-domain antibody phage library: add 100µL of glycerol bacterial library to 2×YT medium, when OD600=0.5, add 20 MOI helper phage, let stand for 30min, and centrifuge the pellet with 2×YT medium. Resuspend in YT medium, culture for 1 hour, add antibiotics for 16 hours and then centrifuge, the supernatant is precipitated by pre-cooled PEG-NaCl (1 / 4 volume), and resuspended in 1 mL of PBS is the amplified single domain antibody library;
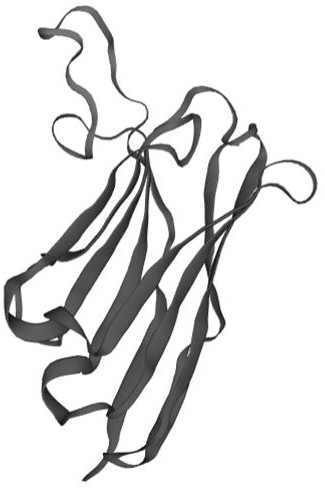
[0038] (2) Immune tube panning: E2 protein ( figure 1, biotin-labeled and purified) 50µg / tube coat the immunotube overnight, remove the coating solution, wash 3 times, block with 2mL BSA (1%) for 2h, wash 3 times with PBST, add 100µL (1) step amplification The single-domain antibody library was used as the primary antibody at 37°C for 2 h, washed with PBST for 3 times, eluted with Glycine-HCI (PH2.2), and...
Embodiment 2
[0041] Example 2: ELISA identification of single clones
[0042] (1) Panning a single positive clone: Inoculate the natural single domain antibody library of the third round of panning in 2×YT medium, OD 600nm = 0.5, add 20 MOI helper phage, let stand for 30 min, resuspend the pellet in 2×YT medium after centrifugation, culture for another 1 h, then spread on 2×YT plate containing antibiotics, culture overnight, select 40 A single colony was inoculated into 2×YT medium. When OD600=0.5, 20 MOI of helper phage was added and left for 30 min. After centrifugation, the pellet was resuspended in 2×YT medium, re-cultured, and IPTG was added to induce expression for 8 h. .
[0043] (2) ELISA identification: 100µL / well of E2 protein (with biotin tag and purified, concentration 1ng / µL) was coated on ELISA plate overnight, washed 3 times after removing the coating solution, and blocked with 200µL / well BSA (3%). 2h, washed 3 times with PBST, added 100µL / well of the single domain antib...
Embodiment 2
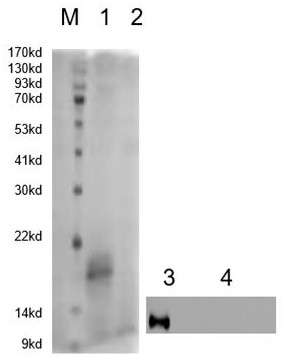
[0045] Example 2 Specific identification of HCV-E2
[0046] The above sequence was sent to BGI (Beijing) to purify and synthesize the E2 protein single-domain antibody HCV-E2 for Western blot identification: A. Protein electrophoresis: Dilute the purified E protein with 6 x SDS protein electrophoresis loading buffer To 60μg, boil at 100 ℃ for 5 min, pre-stain Marker 2 μL, carry out protein electrophoresis on concentrated gel at 80 v and separation gel at 120 v. B. Transfer membrane: Put the gel on the nitrocellulose membrane (NC membrane), put 3 Whatman 3 mm filter papers on the top and bottom, put the above items in the transfer membrane electrophoresis buffer and soak for 15 minutes to drive out the residue on the filter membrane. on the bubbles. Install the electrotransfer device in sequence, place 3 pieces of filter paper, gel, NC membrane, and 3 pieces of filter paper in sequence on the negative electrode plate to ensure the precise alignment of each layer (from bottom t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com