Electrode for carbon dioxide electroreduction and preparation method thereof
A carbon dioxide, electrode technology, applied in the direction of electrodes, electrolysis components, electrolysis process, etc., to achieve high electroreduction activity and stability, improve current density, and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
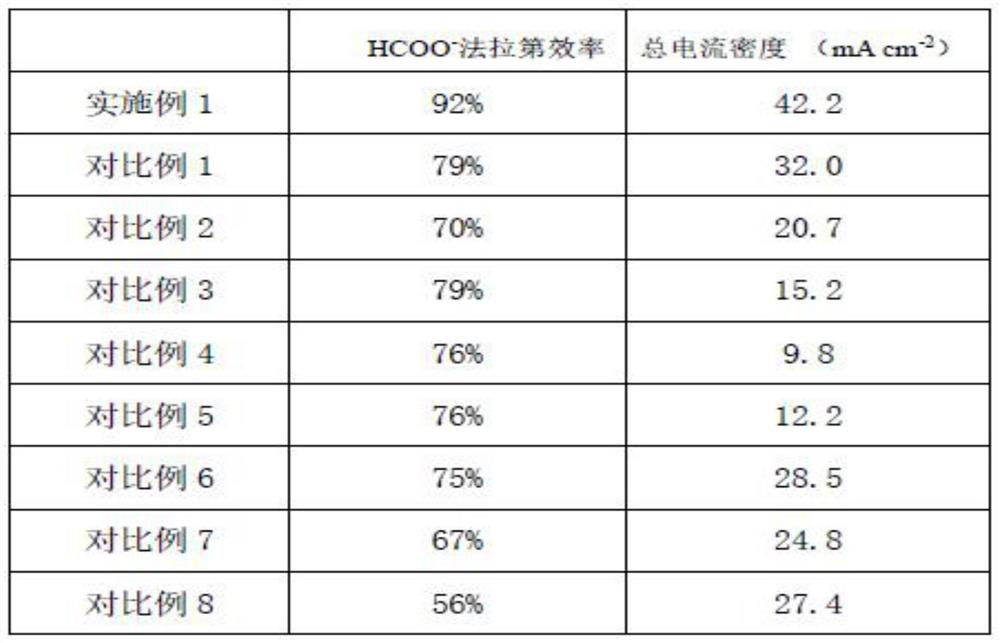
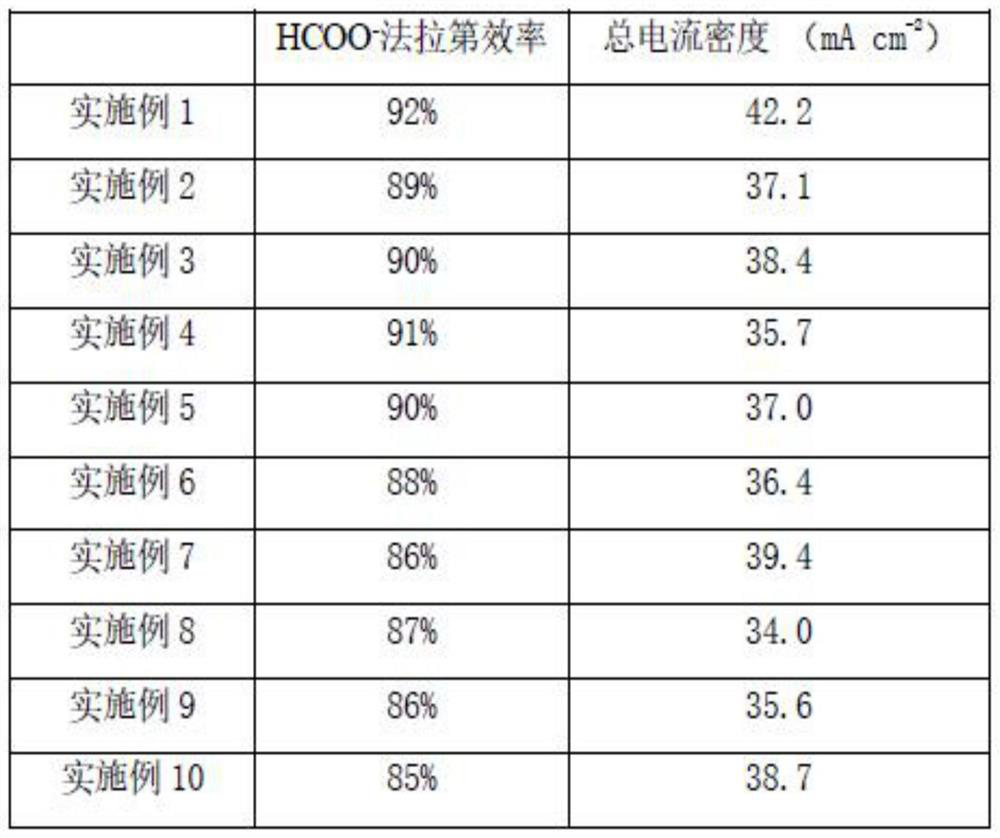
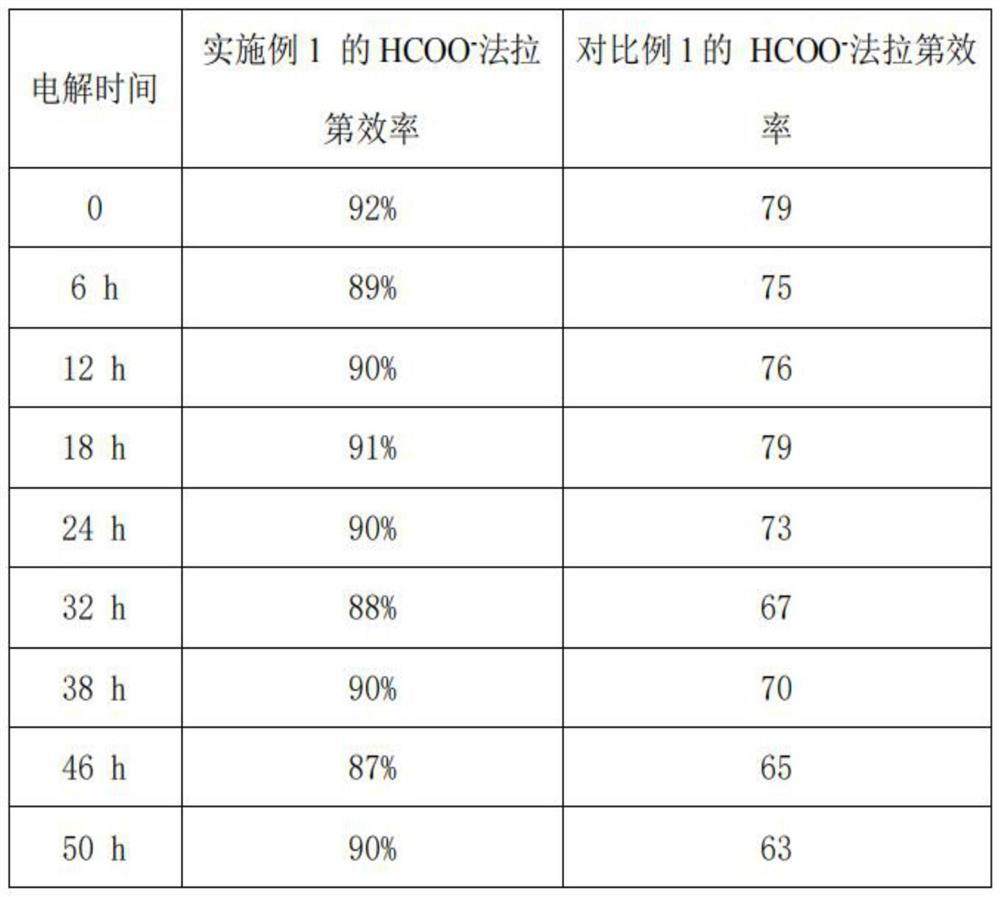
Embodiment 1
[0035] A 200 μm copper mesh was used as the base, cleaned with acetone and hydrochloric acid, and then placed in NH 3 ·H 2 O and (NH 4 ) 2 S 2 O 8 The oxidizing solution with a molar ratio of 8:1 was treated for 8 min. After cleaning, it is used as the base layer; the amino cellulose solution with a concentration of 0.2% is prepared, and diethylamino cellulose is sprayed by spraying method to the surface of the base layer, and the loading amount of the transition layer is 0.3 mg / cm 2 . The electrodes after spraying were placed in a vacuum oven to dry at 100 °C for 6 h. SnCl 2 Mixed with hydrochloric acid and water to prepare SnCl with a concentration of 0.2mol / L 2 The aqueous solution is mixed with 0.6 mol / L NaF and 0.10 mol / L ethylenediaminetetraacetic acid, and then 0.09 mol / L sodium citrate is added to obtain an electroplating solution, and the pH value is adjusted at 4 by using hydrochloric acid. The molar ratio of Sn to EDTA is 8:1 and the molar ratio of NaF to S...
Embodiment 2
[0053] A 200 μm copper mesh was used as the base, cleaned with acetone and hydrochloric acid, and then placed in NH 3 ·H 2 O and (NH 4 ) 2 S 2 O 8 The oxidizing solution with a molar ratio of 8:1 was treated for 8 min. After cleaning, it is used as the base layer; the amino cellulose solution with a concentration of 0.2% is prepared, and p-aminobenzyl cellulose is sprayed on the surface of the base layer, and the loading amount of the transition layer is 0.3 mg / cm 2 . The electrodes after spraying were placed in a vacuum oven to dry at 100 °C for 6 h. SnCl 2 Mixed with hydrochloric acid and water to prepare SnCl with a concentration of 0.2mol / L 2 The aqueous solution is mixed with 0.6 mol / L NaF and 0.10 mol / L iminodiacetic acid evenly, then 0.09 mol / L sodium citrate is added to obtain an electroplating solution, and the pH value is adjusted at 4 with hydrochloric acid. The molar ratio of Sn to iminodiacetic acid is 8:1 and the molar ratio of NaF to Sn is 15:1. Under ...
Embodiment 3
[0055] A 200 μm copper mesh was used as the base, cleaned with acetone and hydrochloric acid, and then placed in NH 3 ·H 2 O and (NH 4 ) 2 S 2 O 8 The oxidizing solution with a molar ratio of 8:1 was treated for 8 min. After cleaning, it is used as the base layer; the amino cellulose solution with a concentration of 0.2% is prepared, and diethylamino cellulose is sprayed by spraying method to the surface of the base layer, and the loading amount of the transition layer is 0.5 mg / cm 2 . The electrodes after spraying were placed in a vacuum oven to dry at 100 °C for 6 h. SnCl 2 Mixed with hydrochloric acid and water to prepare SnCl with a concentration of 0.2mol / L 2 The aqueous solution is mixed with 0.6 mol / L NaF and 0.10 mol / L iminodiacetic acid evenly, then 0.09 mol / L sodium citrate is added to obtain an electroplating solution, and the pH value is adjusted at 4 with hydrochloric acid. The molar ratio of Sn to EDTA was 8:1, and the molar ratio of NaF to Sn was 15:1. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com