Method for transduction of cells by viral vectors
A virus carrier and cell technology, applied in the field of genetic engineering, can solve the problems of delaying the timing of tumor treatment, affecting the clinical treatment effect, and taking a long time for T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0132] In some embodiments, the method of preparation includes the step of freezing (eg, cryopreserving) the cells before or after isolation, selection and / or enrichment and / or incubation for transduction and engineering. In some embodiments, the freezing and subsequent thawing steps remove granulocytes, and to some extent monocytes, in the cell population. In some embodiments, the cells are suspended in a freezing solution to remove plasma and platelets, such as after a washing step. In some aspects, any of a variety of known freezing solutions and parameters can be used. An example involves the use of PBS containing 20% DMSO and 8% human serum albumin (HSA), or other suitable cell freezing medium. It was then diluted 1:1 with medium so that the final concentrations of DMSO and HSA were 10% and 4%, respectively. Cells are then typically frozen to -80°C at a rate of 1° / min and stored in the gas phase of a liquid nitrogen storage tank.
[0133] In some embodiments, isolati...
Embodiment 1
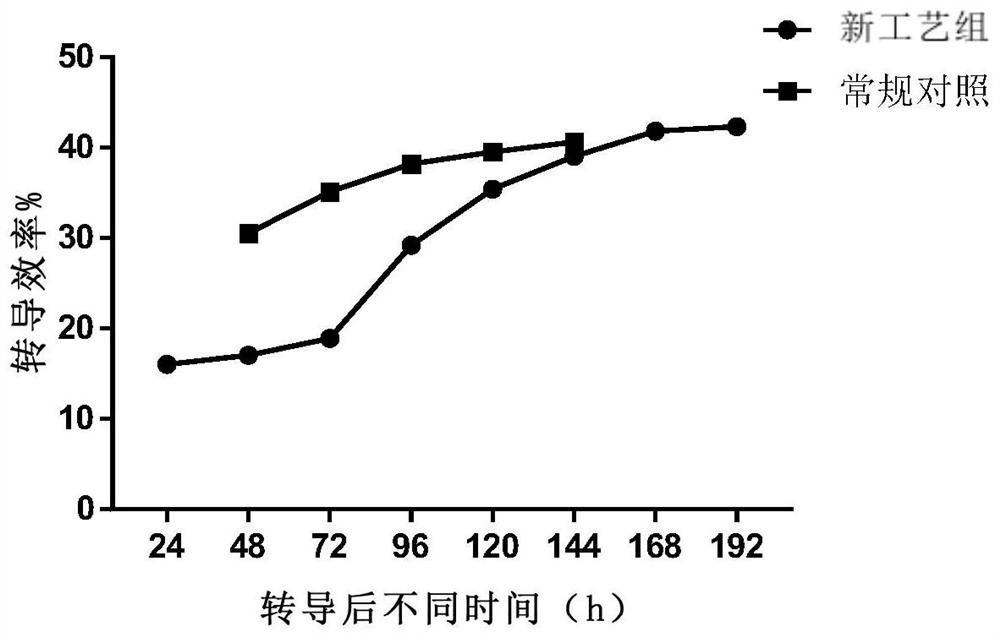
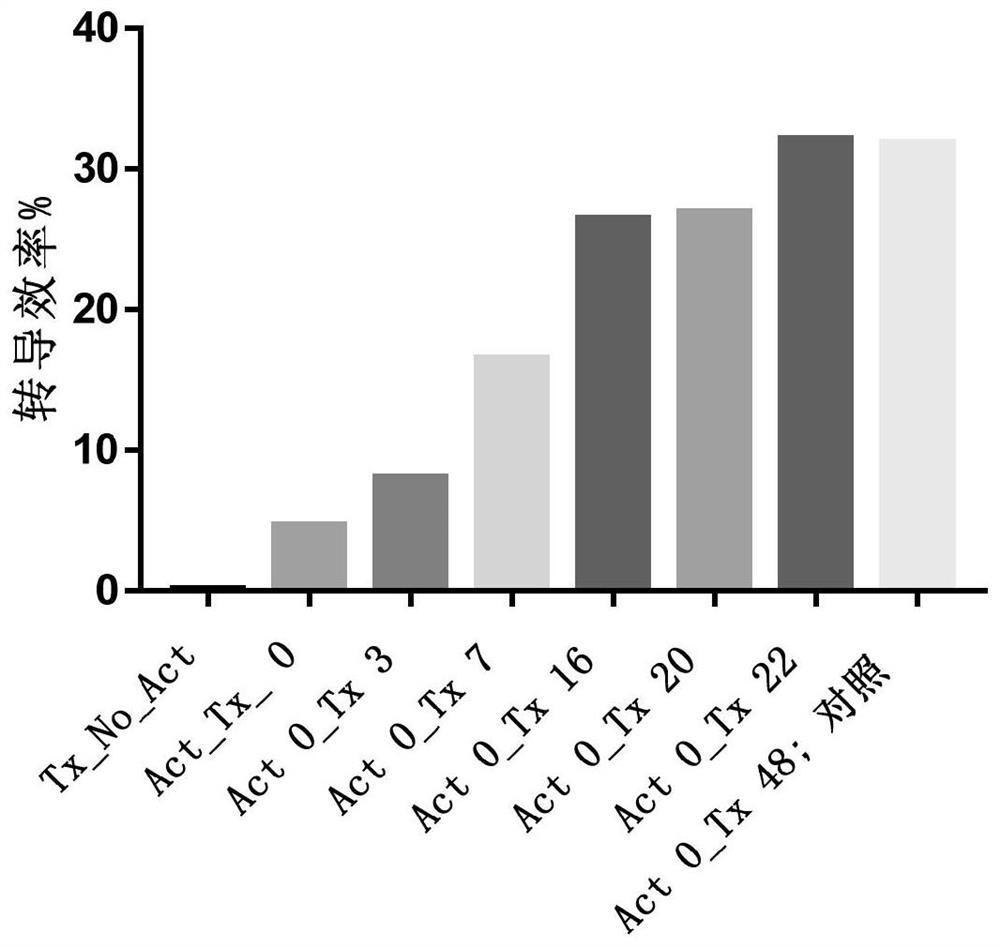
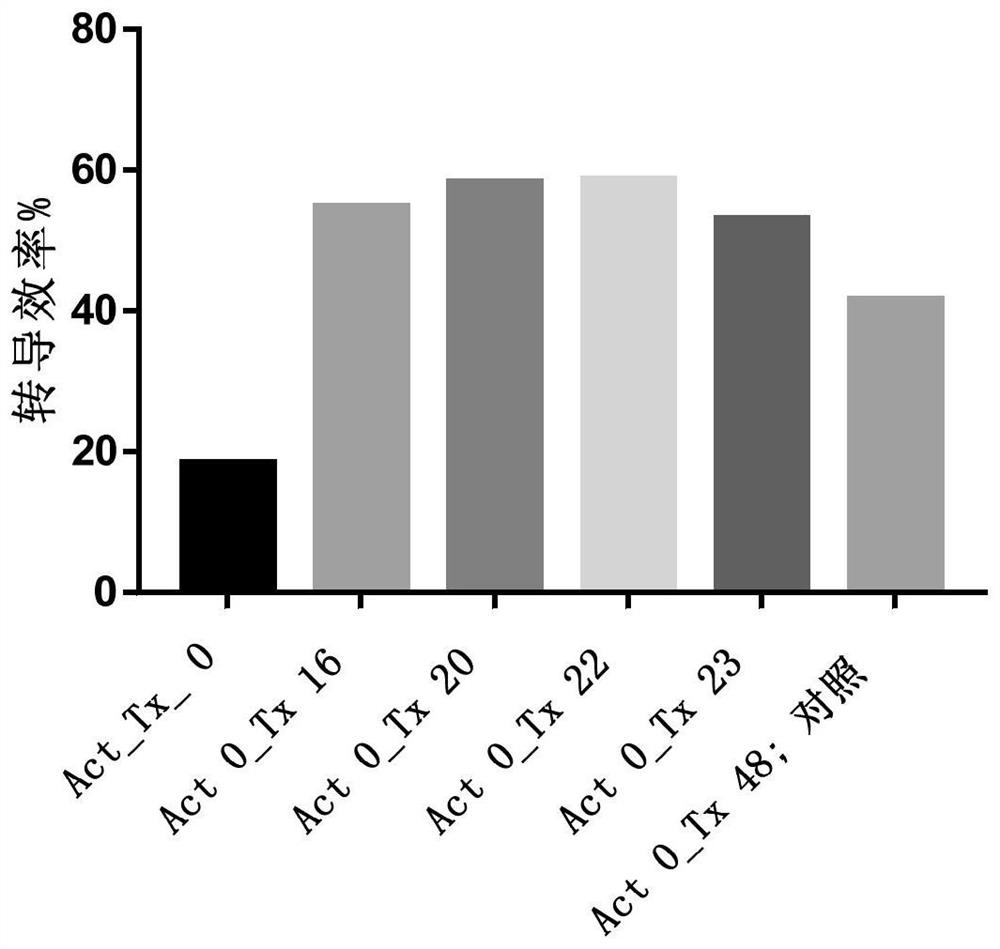
[0512] Example 1. Evaluation of Lentiviral Transduction Efficiency
[0513] Leukocyte-rich samples were collected from subjects using leukapheresis, and buffy coat was collected using Ficoll density gradient centrifugation to obtain peripheral blood mononuclear cells (PBMCs) with high purity.
[0514] PBMCs were washed and resuspended in buffer containing phosphate buffered saline (PBS), EDTA and human serum albumin, washed cells in sorting buffer were mixed with commercially available magnetic beads coupled to monoclonal antibodies The reagents were incubated together for 30 minutes at room temperature and sorted using a magnetic separation column to obtain an enriched population of T cells. The resulting enriched T cell population was resuspended in X-VIVO 15 medium (purchased from Lonza), and T cell activator was added (conjugated to anti-CD3 and / or lentiviral vector particles) Solid supports for anti-CD28 and / or anti-41-BB monoclonal antibodies (e.g., beads, including mag...
Embodiment 2
[0529] Example 2: Evaluation of Transduction Efficiency of Primary T Cell Activated Transduction Culture for 24-48 Hours
[0530] Leukocyte-rich samples were collected from subjects using leukapheresis, and buffy coat was collected using Ficoll density gradient centrifugation to obtain peripheral blood mononuclear cells (PBMCs) with high purity.
[0531] Aseptically transfer the obtained PBMCs into transfer bags. PBMCs were washed and resuspended in selection buffer containing PBS, EDTA and human serum albumin for affinity-based selection. Washing was performed in a sterile single-use disposable kit for regenerative medicine sold by Biosafe SA, which includes a centrifuge chamber (A-200F). The transfer bag containing the cells and the bag containing the buffer were aseptically attached to the kit, which was placed in connection with the Sepax2 processing unit. Two (2) wash cycles were performed, each with approximately 200 g of RCF at the inner wall of the cavity for 180 sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com