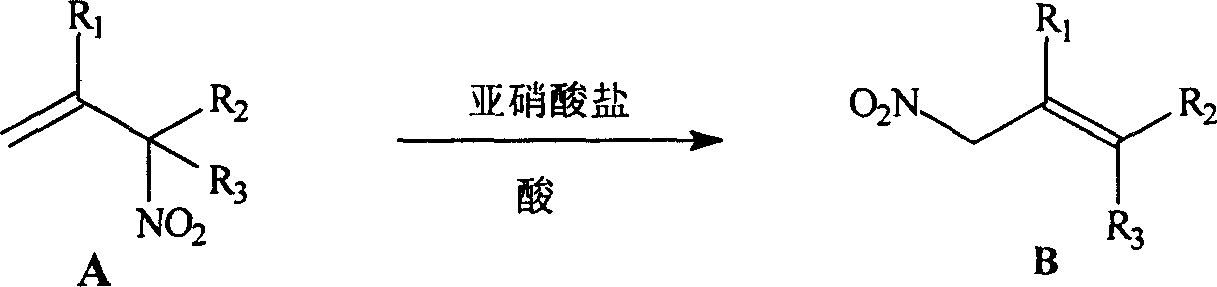
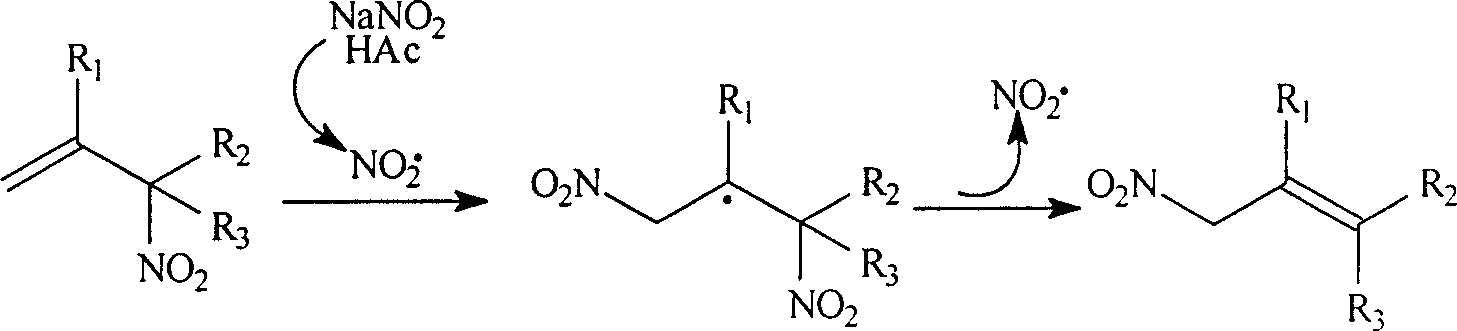
Process for preparing allylic primary nitro compound
A technology of nitro compound and primary allylic position is applied in the field of preparation of primary nitro compound at allylic position, which can solve the problems of long reaction time, high toxicity and the like, and achieve simple steps, low corrosiveness, and economical and easy-to-obtain raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
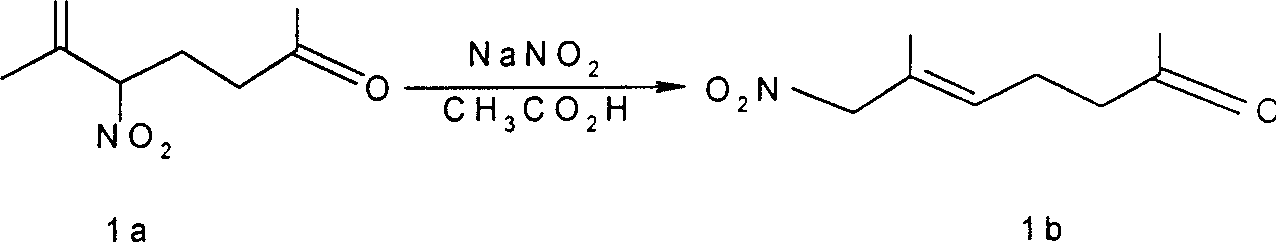
[0011] Example 1 From 5-nitro-6-methyl-6-hepten-2-one ( 1a ) to prepare 7-nitro-6-methyl-5-hepten-2-one ( 1b ), the reaction formula is as follows:
[0012]
[0013] Reaction formula (1)
[0014] Synthesis steps: introduce nitrogen oxide gas generated by mixing 10g (145mmol) sodium nitrite and 15ml (260mmol) glacial acetic acid into 30mL carbon tetrachloride, and add the resulting carbon tetrachloride solution dropwise to Contains 1.24g (7.3mmol) nitroketone 1a In 30ml of carbon tetrachloride, continue to stir at 60 ° C for 1 hour, then evaporate the solvent, and the crude product is obtained by silica gel column chromatography (elution condition: 2 / 8 ether / petroleum ether) 1b , 1.02g, yield 82%.
[0015] Infrared spectral analysis (IR, cm -1 , liquid film): 1716 (C=O), 1552 (NO 2 ).
[0016] NMR (90MHz) analysis:
[0017] δ H 5.54(t, 1H, CH=C), 4.76(s, 2H, -CH 2 NO 2 ), 1.76 (s, 3H, CH 3 C=C), 2.12(s, 3H, CH 3 C=O), 2.24-2.64 (m, 4H, -CH ...
Embodiment 2
[0018] Embodiment 2 By 5-nitro-5-acetic acid hydroxymethyl-6-methyl-6-hepten-2-ketone ( 2a ) to prepare 7-nitro-5-acetic acid hydroxymethyl-6-methyl-5-hepten-2-ketone ( 2b ). The reaction formula is as follows:
[0019]
[0020] Reaction formula (2)
[0021] Synthesis steps: 10g (145mmol) sodium nitrite and 15ml (260mmol) glacial acetic acid are mixed to generate nitrogen oxide gas into 25ml carbon tetrachloride, and the resulting carbon tetrachloride solution is added to the containing 1.16g (5mmol) nitroester ketone 2a In 30mL of carbon tetrachloride, continue to stir at 60°C for 1 hour, then evaporate the solvent, and the crude product is chromatographed on a silica gel column (elution condition: 1 / 2 ethyl ether / petroleum ether) to obtain 2b, 0.89g, and rate of 77%.
[0022] Infrared spectral analysis (IR, cm -1 , liquid film): 1743 (C=O, ester), 1714 (C=O, ketone), 1655 (C=C), 1558 (NO 2 ), 1234, 1048.
[0023] NMR (90MHz) analysis:
[0024]...
Embodiment 3
[0025] Embodiment 3 By 6-nitro-3,7-dimethyl-7-octenal ( 3a ) to prepare 8-nitro-3,7-dimethyl-6-octenal ( 3b )
[0026] The reaction formula is as follows:
[0027]
[0028] Reaction formula (3)
[0029] Synthesis steps: introduce nitrogen oxide gas generated by mixing 6.7g (100mmol) sodium nitrite and 10mL (174mmol) glacial acetic acid into 15ml carbon tetrachloride, and add the resulting carbon tetrachloride solution dropwise at 55-60°C To contain 0.95g (5mmol) nitroaldehyde 3a In 15ml of carbon tetrachloride, continue to stir at 60 ° C for 1 hour, then evaporate the solvent, and the crude product is obtained by silica gel column chromatography (elution condition: 1 / 3 ether / petroleum ether) 3b , 0.66g, yield 69%.
[0030] Infrared spectral analysis (IR, cm -1 , liquid film): 1724 (C=O), 1552 (NO 2 ).
[0031] NMR (300MHz) analysis: δ H : 9.76 (d, 1H, CHO), 5.63 (t, 1H, C=CH), 4.83 (s, 2H, -CH 2 NO 2 )1.77 (s, 3H, CH 3 ), 0.99 (d, CH 3 , CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com