Parathyroid hormone analogues for the treatment of osteoporosis
A technology of parathyroid hormone and analogues, applied in the field of analogues of human parathyroid hormone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
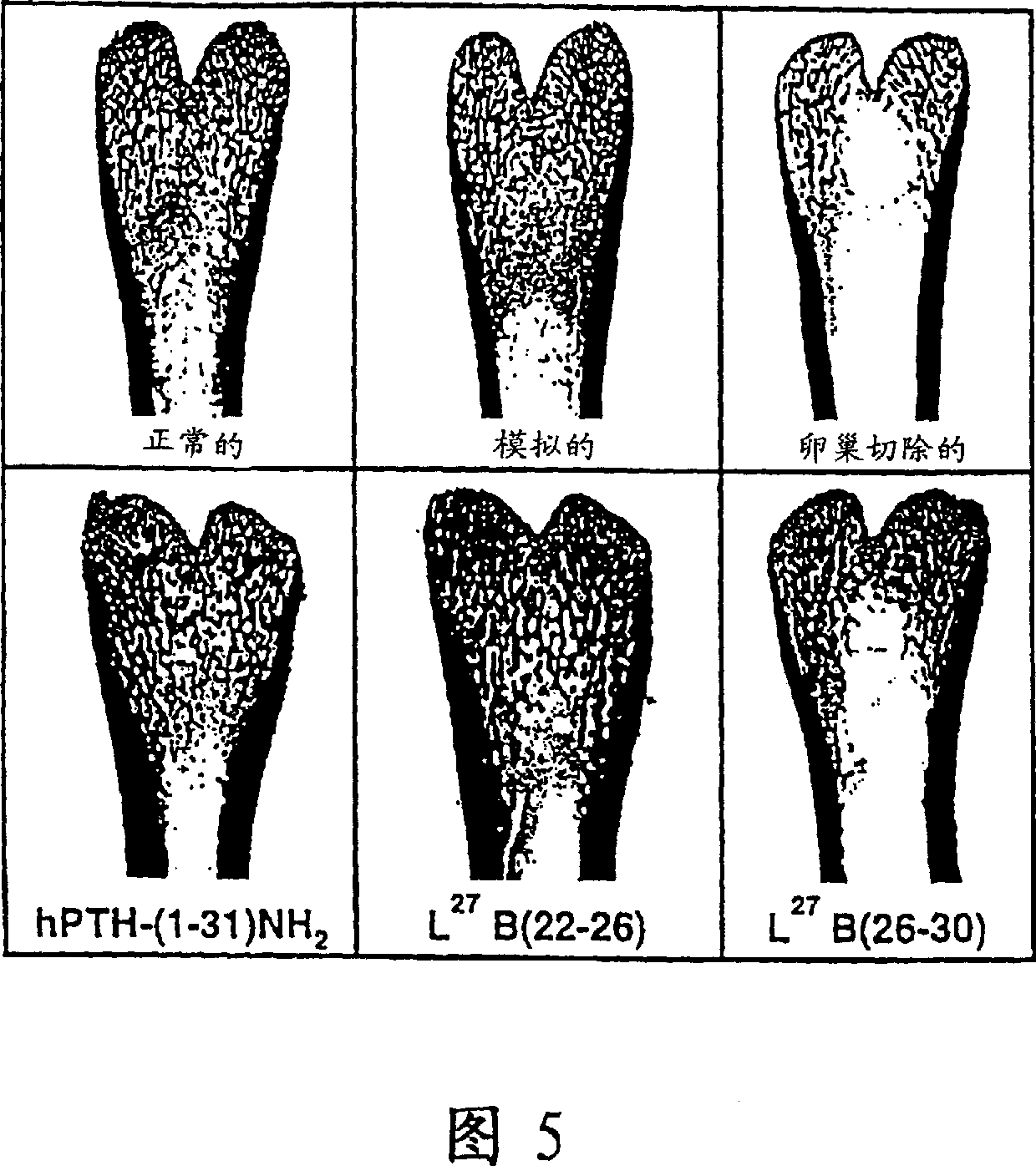
Image
Examples
Embodiment 1
[0086] Example 1 Synthesis and purification of linear hPTH-(1-31) amide analogs
[0087] During the coupling process, the α-amino group of the amino acid is protected by 9-fluorenylmethoxycarbonyl (Fmoc). With 1-hydroxybenzotriazole (HOBt), 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU) and diiso A mixture of propylethylamine (DIPEA) was used for coupling. Asn, Gln, His, Val and Ile residues were added by double coupling using a 4-fold excess of the activated amino acid. The coupling time was increased from 30 minutes to 60 minutes with the addition of Arg and Gly. The first residue (Val 31 ) coupled to the carrier (Tentagel * R, Rapp Polymere, Tubingen, Germany). All other steps in PerSeptive Biosystems * Model 9050 Plus automatic peptide synthesizer. The side chains are protected as follows: Arg (2,2,5,7,8-pentamethylchroman-6-sulfonyl); Glu, Asp and Ser (t-butyl); His, Gln and Asn (trityl); Trp (t-butoxycarbonyl).
[0088] After remova...
Embodiment 2
[0090] Example 2 Synthesis and purification of cyclic analogs
[0091] [Leu 27 ] Ring (Glu 22 -Lys 26 )-hPTH-(1-31)-NH 2 Peptides were synthesized as described in Example 1. Lys-Alloc and Glu-OAII were substituted at positions 26 and 22, respectively. Join Fmoc-Ser 17 Afterwards, the peptide-resin was transferred from the column to a reaction tube (Minivial * , AppliedScience), and suspended under argon in 1.7 ml dichloromethane (DCM) containing tetrakis (triphenylphosphine) palladium (0.24 mmol), 5% acetic acid and 2.5% N-methylmorpholine (NMM) solution, followed by shaking at 20°C for 6 hours to remove the allyl and alloc protecting groups [Sole, N.A. et al. (1993), in Peptides: Chemistry, Structure and Biology, Smith, J. and Hodges, R.( eds), ESCOM, pp. 93-94, cited here by reference]. The peptide-resin was then washed with DMF (50ml) containing 0.5% diethyldithiocarbamate (DEDT), 0.5% NMM, followed by DMF (50ml) and DCM (50ml). The peptide (0.06 mmol) was cyclized...
Embodiment 3
[0093] Example 3 Determination of adenylyl cyclase
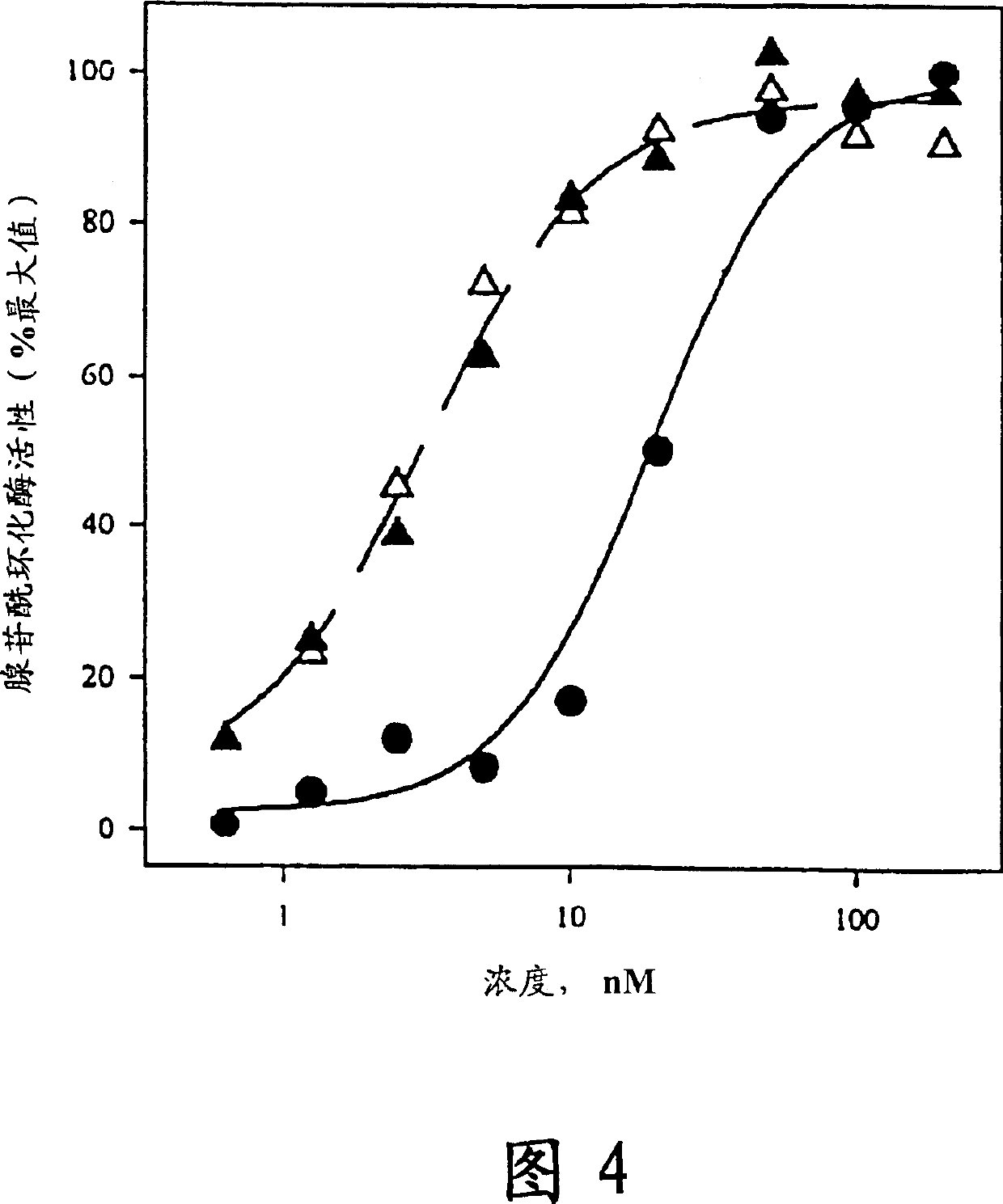
[0094] The ability of hPTH to mimic a signaling mechanism coupled to receptor binding and its activation of adenylyl cyclase was assayed on the differentiation-competent osteoblast-like ROS17 / 2 rat osteosarcoma (RUS) cell line. This activity is known to be tightly coupled with the analog's ability to remodel bone mass in ovariectomized rats. Stimulate the activity of adenylyl cyclase by using [ 3 [H]-adenine pre-labels cellular ATP pools and then measures the 10 min period beginning after exposure to the specific analog by [ 3 H]-ATP produced [ 3 H]-cyclic AMP was evaluated. This is based on the method described in Whitfied et al., J. Cell Physiol., 150, 299-303, 1992, incorporated herein by reference.
[0095] The adenylyl cyclase results are presented in Table 2 below as the concentration required to achieve a half-maximal increase in AC activity. This data is also presented in Figure 4. In Figure 4, the solid circle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com