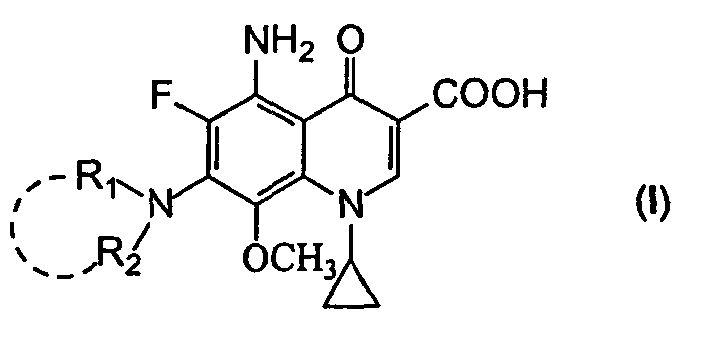
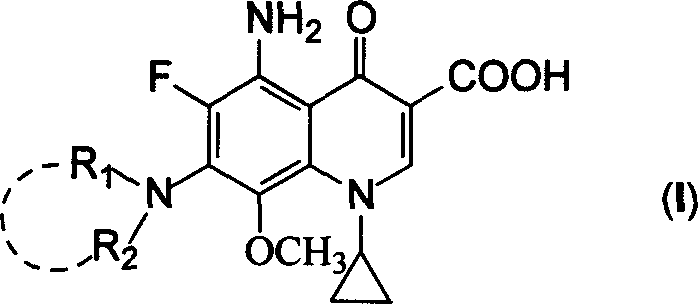
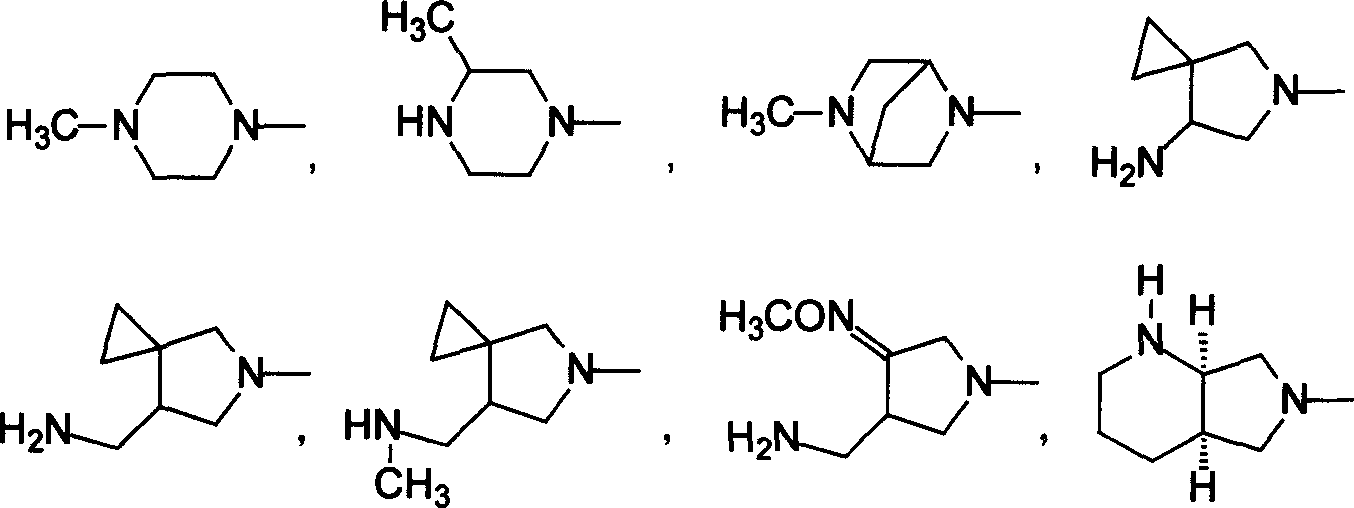
5-amino-8-methoxy quinolone carboxylic acid derivatives and its preparation
A kind of amino protecting group and compound technology, applied in the field of 5-amino-8-methoxyquinolone carboxylic acid derivatives and preparation methods thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0130] Preparation of 1-cyclopropyl-6,7-difluoro-8-methoxy-5-nitro-1,4-dihydro-4-oxoquinoline-3-carboxylic acid ethyl ester
[0131] Under ice bath, ethyl 1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylate (10.0g, 30.9mmol) Soluble in concentrated H 2 SO 4 (85.0mL), add KNO in batches 3 (4.7g, 46.4mmol) and reacted at the same temperature for 3h. After the reaction was completed, the reaction solution was poured into rapidly stirred ice water (500 mL), continued to stir for 0.5 h, filtered, and the filter cake was dried and recrystallized from methanol to obtain VII-1 (9.5 g, 83.3%) as a white solid. mp>270°C.
[0132] 1 H NMR (CDCl 3 )δ, ppm: 1.07-1.24 (4H, m), 1.36 (3H, t, J = 7.8Hz), 4.02-4.04 (1H, m), 4.17 (3H, s), 4.35 (2H, q, J = 7.8Hz), 8.60(1H, s)
preparation example 2
[0134] Preparation of 5-amino-1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid ethyl ester
[0135] Reduced iron powder (2.4g, 42.8mmol), H 2A mixture of O (4.5mL) and glacial acetic acid (0.3mL) was reacted under reflux for 15min, a chloroform (100mL) solution of the compound (4.0g, 10.8mmol) prepared in Preparation Example 1 was added dropwise, and the reflux reaction was continued for 6h, and then After filtering, the filtrate was left to cool and washed twice with 6M HCl. Dry, filter, remove the solvent from the filtrate to obtain a crude product, recrystallize from a mixed solvent of ethanol and chloroform to obtain a yellow solid (3.2 g, 87.2%), mp 209-210°C.
preparation example 3
[0137] Preparation of 5-amino-1-cyclopropyl-6,7-difluoro-8-methoxy-1,4-dihydro-4-oxoquinoline-3-carboxylic acid
[0138] The compound (3.2g, 9.5mmol) prepared in Preparation Example 2 was dissolved in concentrated HCl (4.0mL) and HOAc (16.0mL), reacted at 100°C for 1h, concentrated the reaction solution to about 5.0mL, and poured the residue into 100mL of ice water, stirred for 0.5h and then filtered, the filter cake was washed with H 2 O was washed and dried to give a yellow solid (2.8g, 93.1%), mp>270°C
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com