Chemical copper plating process for non-aqueous system magnesium base hydrogen storage alloy powder
A technology of hydrogen storage alloy powder and chemical copper plating, applied in the direction of coating, can solve the problems of affecting the copper plating effect alloy performance and other problems, and achieve the effects of reducing the formation of Cu2O, improving corrosion resistance, and inhibiting disproportionation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] With 100ml (wherein methanol accounts for 20%) plating solution, 2g CuSO 4 ·5H 2 O and 5.95g EDTA were dissolved in a 250ml beaker, and 200-400 mesh Mg 1.85 Mmm 0.15 Ni alloy powder 1g, K 4 [Fe(CN) 6 ]·3H 2 O is 2.5mg, using 2ml HCHO as the reducing agent, the stirring speed is 350 rpm, the pH value is 13, and the reaction temperature is 40°C. After the reaction for 15 minutes, the reacted alloy powder is suction-filtered, dried naturally, and sealed. spare.
[0018] The copper-plated alloy powder obtained above was pressed into pieces with a mold, and the non-test surface was coated with epoxy resin, and the electrochemical test was carried out in 5MKOH. The obtained constant potential polarization curve and AC impedance fitting data are shown in Table 1.
[0019] Methanol content
i corr / mA·cm 2
b c / mV
R 1 / Ω·cm 2
R 2 / Ω·cm 2
0
9.6
107
2.87
18.84
20%
6.7
...
Embodiment 2
[0023] With 100ml (wherein methanol accounts for 40%) plating solution, 1.5g CuSO 4 ·5H 2 O and 5.0g EDTA were dissolved in a 250ml beaker, and 200-300 mesh Mg 1.85 Mmm 0.15 Ni alloy powder 1g, K 4 [Fe(CN) 6 ]·3H 2 O is 2.0mg, use 2.5ml HCHO as the reducing agent, adjust the stirring speed to 380 rpm, the pH value to 12.5, and the reaction temperature to 35°C. After reacting for 25 minutes, filter the reacted alloy powder with suction, dry naturally, and seal Packaging, spare.
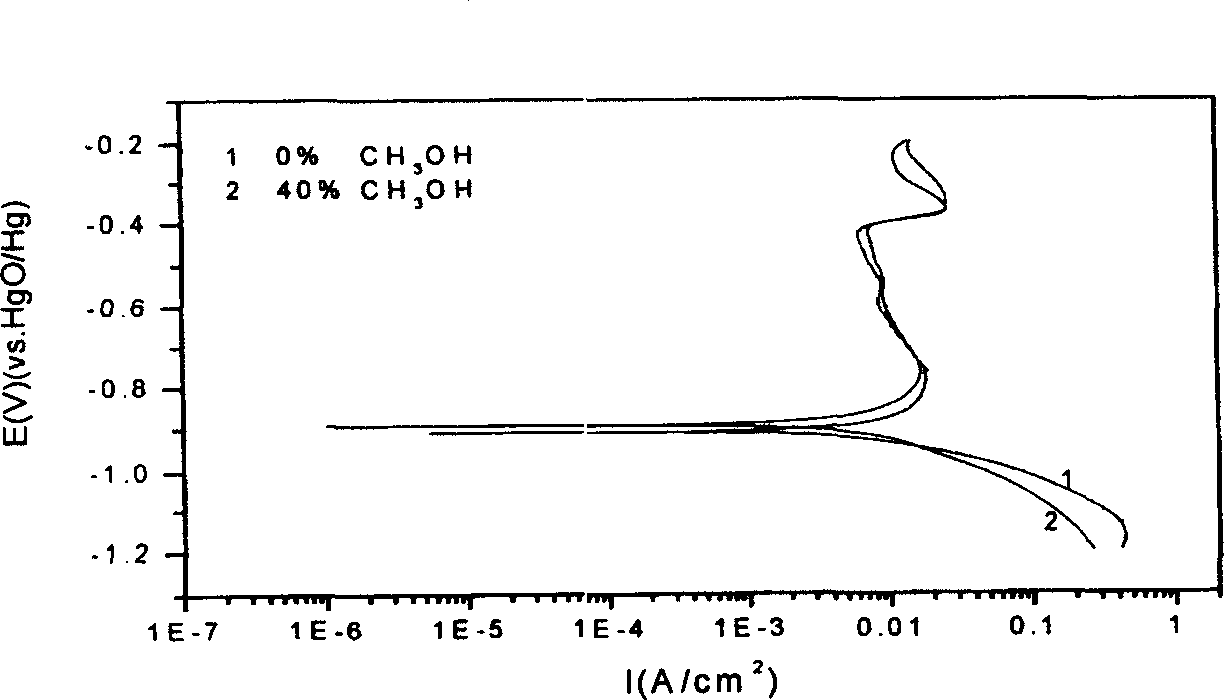
[0024] Press the copper-plated alloy powder obtained above into a sheet with a mold, coat the non-test surface with epoxy resin, and perform an electrochemical test in 5MKOH, and the constant potential polarization curve figure 1 The fitting results with the AC impedance spectrum are shown in Table 2. The copper-plated alloy powder obtained above is carried out SEM test, and the obtained results are shown in figure 2 .
[0025] Methanol content
[0026] Compared with the alloy plate...
Embodiment 3
[0028] With 100ml (wherein methanol accounts for 60%) plating solution, 2.2g CuSO 4 ·5H 2 O and 6.5g EDTA were dissolved in a 250ml beaker, and 200-400 mesh Mg 1.85 Mmm 0.15 Ni alloy powder 2.0g, K 4 [Fe(CN) 6 ]·3H 2 O is 3.1mg, using 2.8ml HCHO as the reducing agent, the stirring speed is 400 rpm, the pH value is 12.8, and the reaction temperature is 45°C. After 19 minutes of reaction, the reacted alloy powder is suction filtered, dried naturally, and sealed. ,spare.
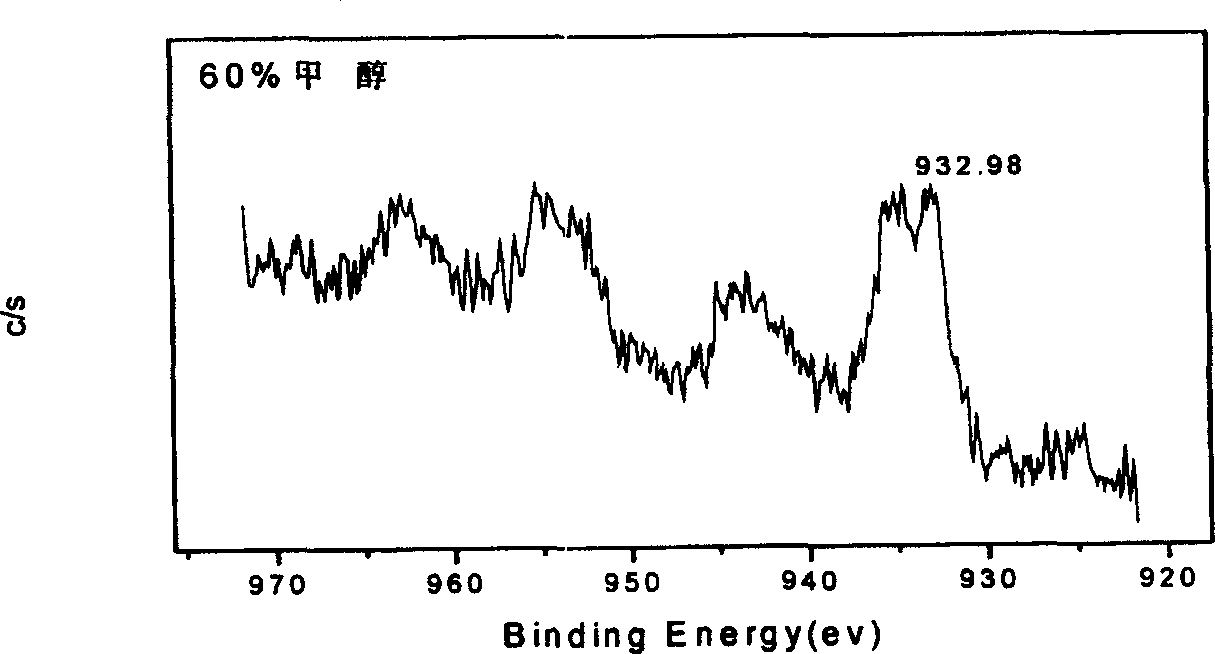
[0029] The copper-plated alloy powder obtained above was pressed into a sheet with a mold, and the non-test surface was coated with epoxy resin, and the electrochemical test was carried out in 5MKOH. The above-mentioned copper-plated alloy powder obtained is carried out XPS test, and the obtained results are shown in image 3 .
[0030] From the test results of the constant potential polarization curve, the alloy powder coated with copper in 60% methanol reduces the corrosion potential and improves the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com