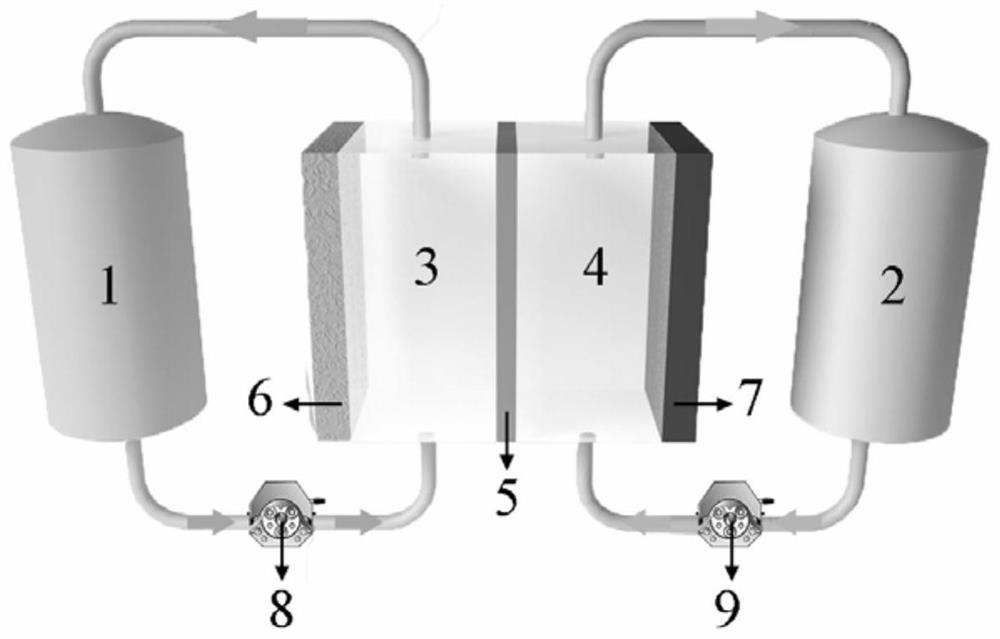
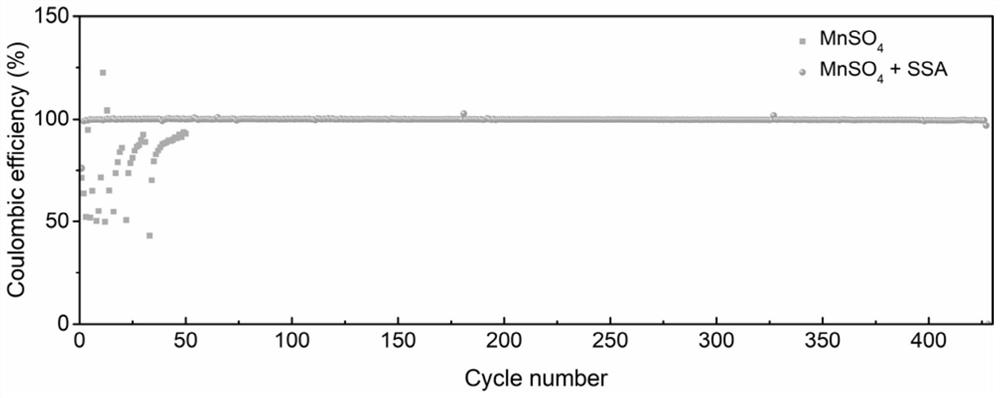
Positive electrode electrolyte for zinc-manganese flow battery
A positive electrolyte and flow battery technology, which is applied in the field of positive electrolyte for zinc-manganese flow batteries, can solve the problems of short life and low battery performance, achieve low cost, improve performance and cycle life, and eliminate manganese dioxide The generated effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In this embodiment, the positive electrode electrolyte of the zinc-manganese redox flow battery includes: manganese sulfate, sulfosalicylic acid with a pH of 5 as an additive, potassium sulfate as a supporting electrolyte, and deionized water as a solvent. Among them, the concentrations of manganese sulfate, sulfosalicylic acid and potassium sulfate are all 0.5mol / L, and the positive and negative electrodes and the proton exchange membrane area are both 2×2cm 2 .
[0035] The specific configuration steps of the positive electrode electrolyte are: weigh 6.35g of sulfosalicylic acid particles in a beaker and dissolve them with deionized water, continuously add potassium hydroxide until the pH is 5, then weigh 4.225g of manganese sulfate and dissolve them with deionized water; Pour the above solution into a 50mL volumetric flask, dilute to 50mL with deionized water and mix well.
[0036] Negative Electrolyte ZnCl 2 Concentration is 0.5mol / L, supporting electrolyte potass...
Embodiment 2
[0042] In this embodiment, in order to further improve the utilization rate of the electrolyte, the positive and negative electrodes and the proton exchange membrane area are selected to be 4×7cm 2 , other conditions are identical with embodiment 1.
[0043] Such as Figure 4 As shown in (a), at 10mA cm -2 When sulfosalicylic acid is added to the manganese sulfate system flow battery under the conditions of current density and charging for 5 minutes, the flow battery can stably cycle 300 times and ensure no decay in Coulombic efficiency. As shown in Figure 4(b), when the current density increases to 20mA cm -2 , and keep charging for 5 minutes, the cycle life of the flow battery at this time is reduced to more than 150 cycles due to the increase in capacity utilization. In general, flow batteries can still maintain stable operation when capacity utilization is increased.
Embodiment 3
[0045] In this embodiment, in order to further improve the cycle life of the battery, other conditions were kept the same as in Embodiment 2, and a constant temperature water bath was used to keep the temperature of the positive and negative electrolytes of the flow battery at 33°C. The result is as Figure 5 shown at 20mAcm -2 When sulfosalicylic acid is added to the manganese sulfate system flow battery under the condition of current density and charging for 5 minutes, the life of the flow battery is 300 cycles, which is doubled compared with the cycle life of the battery at room temperature, while maintaining the energy efficiency of 70 %above.
[0046] The results of the examples show that the positive electrode electrolyte used in the zinc-manganese redox flow battery of the present invention adopts manganese sulfate as the positive electrode active material, sulfosalicylic acid as the additive, potassium sulfate as the supporting electrolyte, and manganese sulfate as th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com