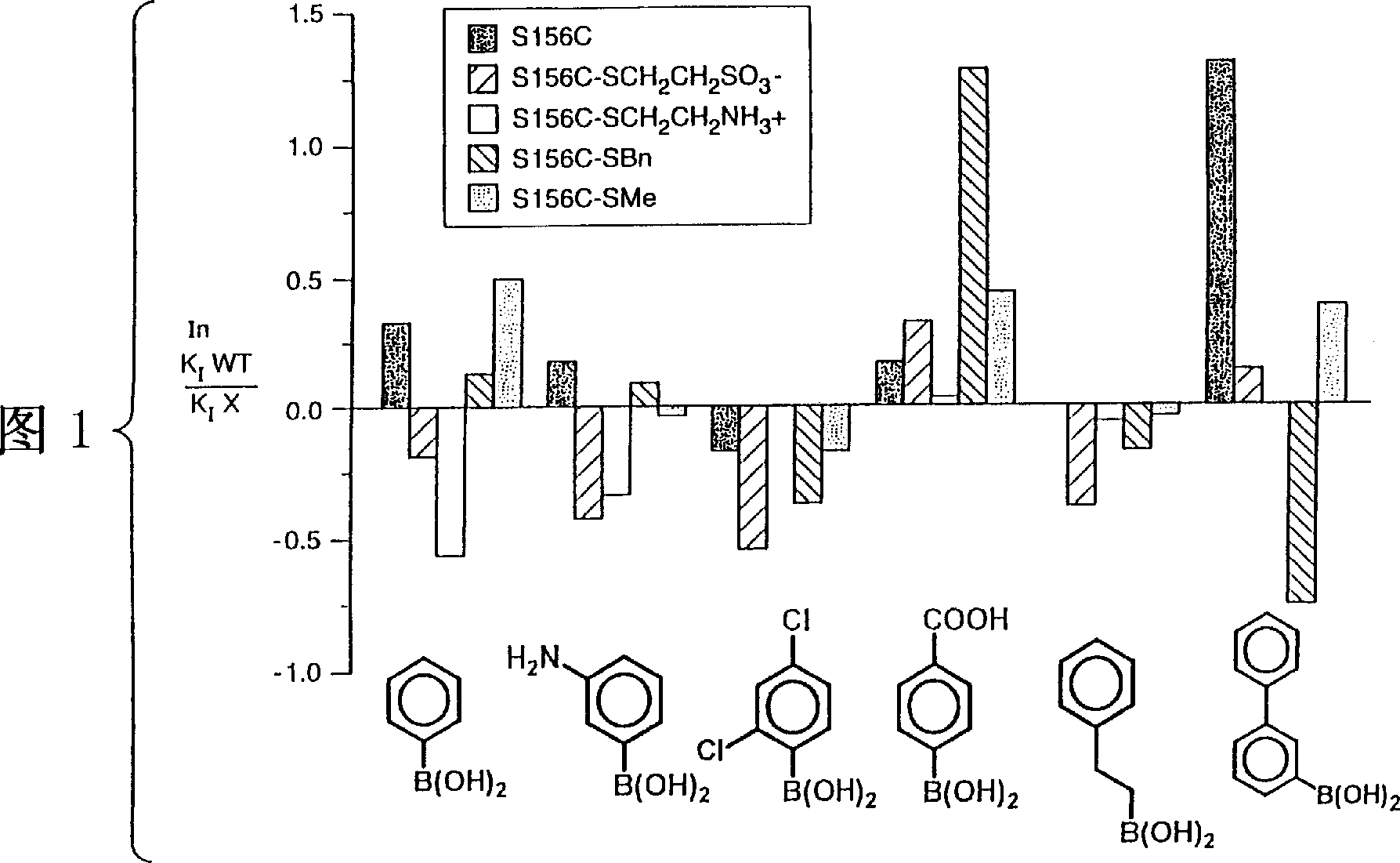
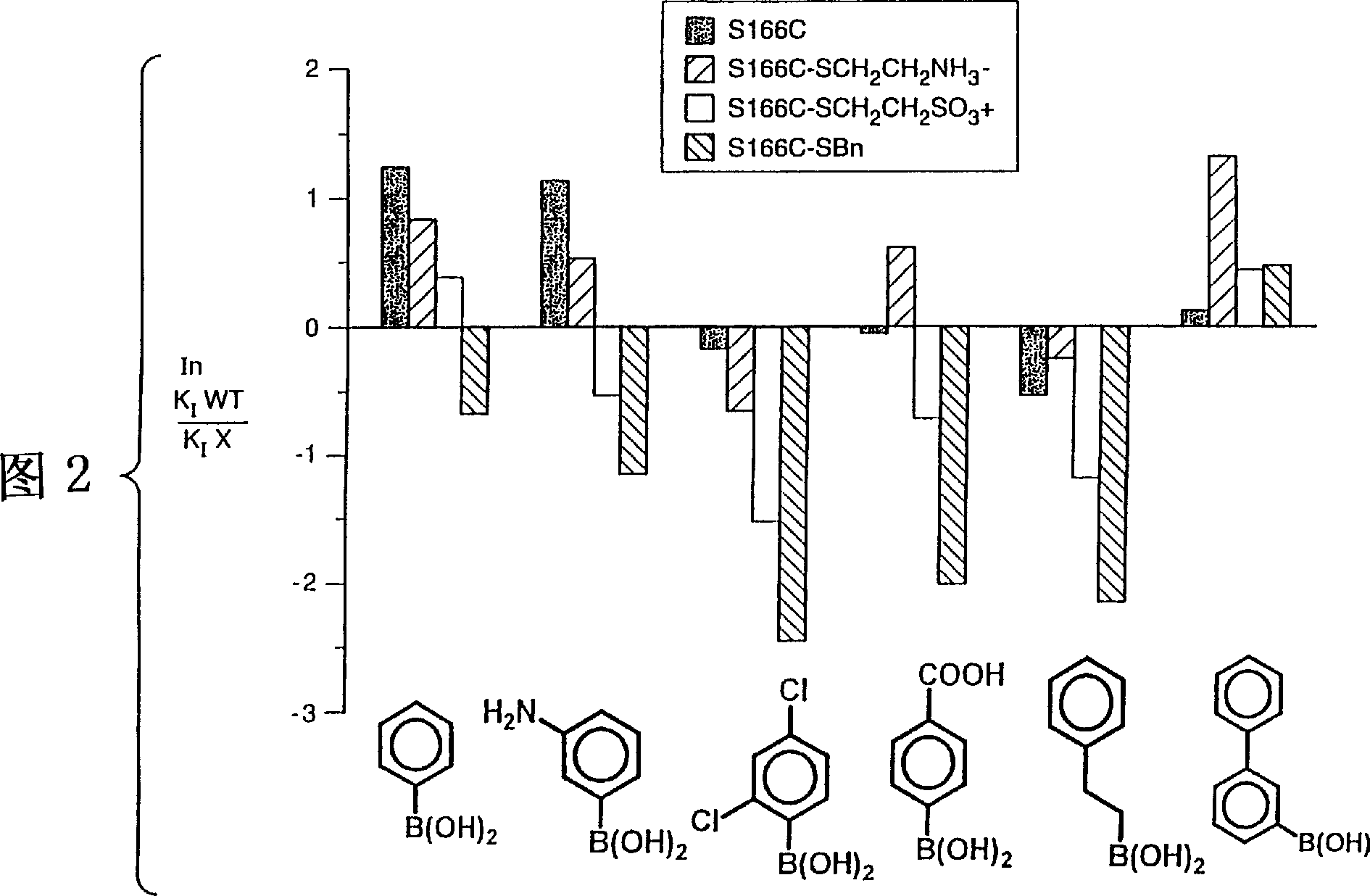
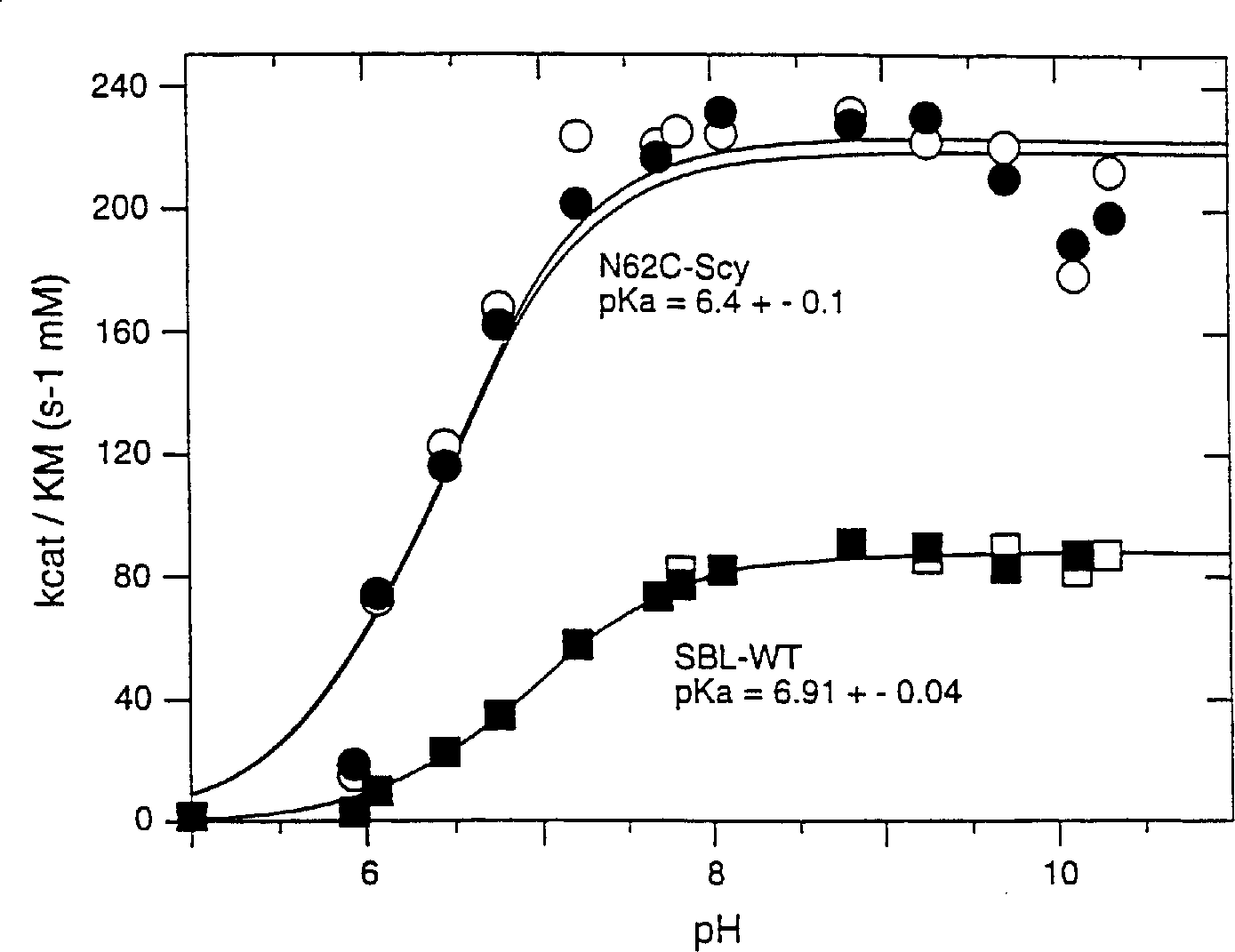
Chemically modified enzymes
A technology for modifying enzymes and proteases, applied in chemical instruments and methods, enzymes, biochemical equipment and methods, etc., can solve the problems that molecular biology methods cannot be easily routinely applied or synthesized in large quantities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The subtilisin (SBL) gene from Bacillus lentus was cloned into the phage M13mp19 vector for mutagenesis (US Patent 5,185,258). Mutagenesis of the indicated oligonucleotides was performed as described in Zoller et al. (1983) Methods in Enzymology, 100: 1468-500. The mutated sequences were cloned, excised and reintroduced into the expression vector GG274 in the Bacillus subtilis host. PEG (50%) was added as a stabilizer. First by using pH 5.2 buffer (20mM sodium acetate, 5mM CaCl 2 ) through Sephadex TM The resulting crude protein concentrate was purified on a G-25 desalting matrix to remove small molecular weight contaminants. The pooled desalted column fractions were then loaded onto a strong cation exchange column (SP Sepharose TM FF), and the SBL was eluted with a gradient of 0-200 mM CaCl acetate buffer (pH 5.2). Salt-free enzyme powder was obtained after dialysis against Millipone pure water followed by lyophilization. Passed with 0.1M HCl 2 The purity of th...
Embodiment 2
[0085] 1-Bromo-3-methylbutane (1.7520 g, 0.0116 mol) and sodium methanesulfonate (1.554 g, 0.0116 mol) in anhydrous DMF (5 ml) were heated at 50° C. for 2 hours. Water (15ml) was added at room temperature and the reaction mixture was extracted with ether (3x30ml). The combined ether extracts were washed twice with brine, dried, concentrated and the residue was subjected to flash column chromatography on silica gel with EtOAc-hexane (1:4). The product was obtained as a colorless liquid (1.4777 g, 70%). IR(film): 3030(w), 3011(w), 2958(st), 2932(st), 2873(st), 1468(m), 1420(w), 1388(w), 1367(w), 1319(st), 1136(st), 955(st), 748cm -1 (st); 1 H NMR (200MHz, CDCl 3 ): δ3.33(s, 3H, CH 3 SO 2 S); 3.19(t, J=7.1Hz, 2H, SCH 2 CH 2 ), 1.70-1.58 (m, 3H, SCH 2 CH 2 CHMe 2 ), 0.95(d, 5.3Hz, 6H, CHMe 2 );13 C NMR (50MHz, CDCl 3 ): δ 50.60, 38.19, 34.59, 27.40, 22.06. Neopentyl Methanesulfonate
[0086] Neopentylsulfonate (3.054g, 0.0154mol), sodium methanesulfonate (2.272g, 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com