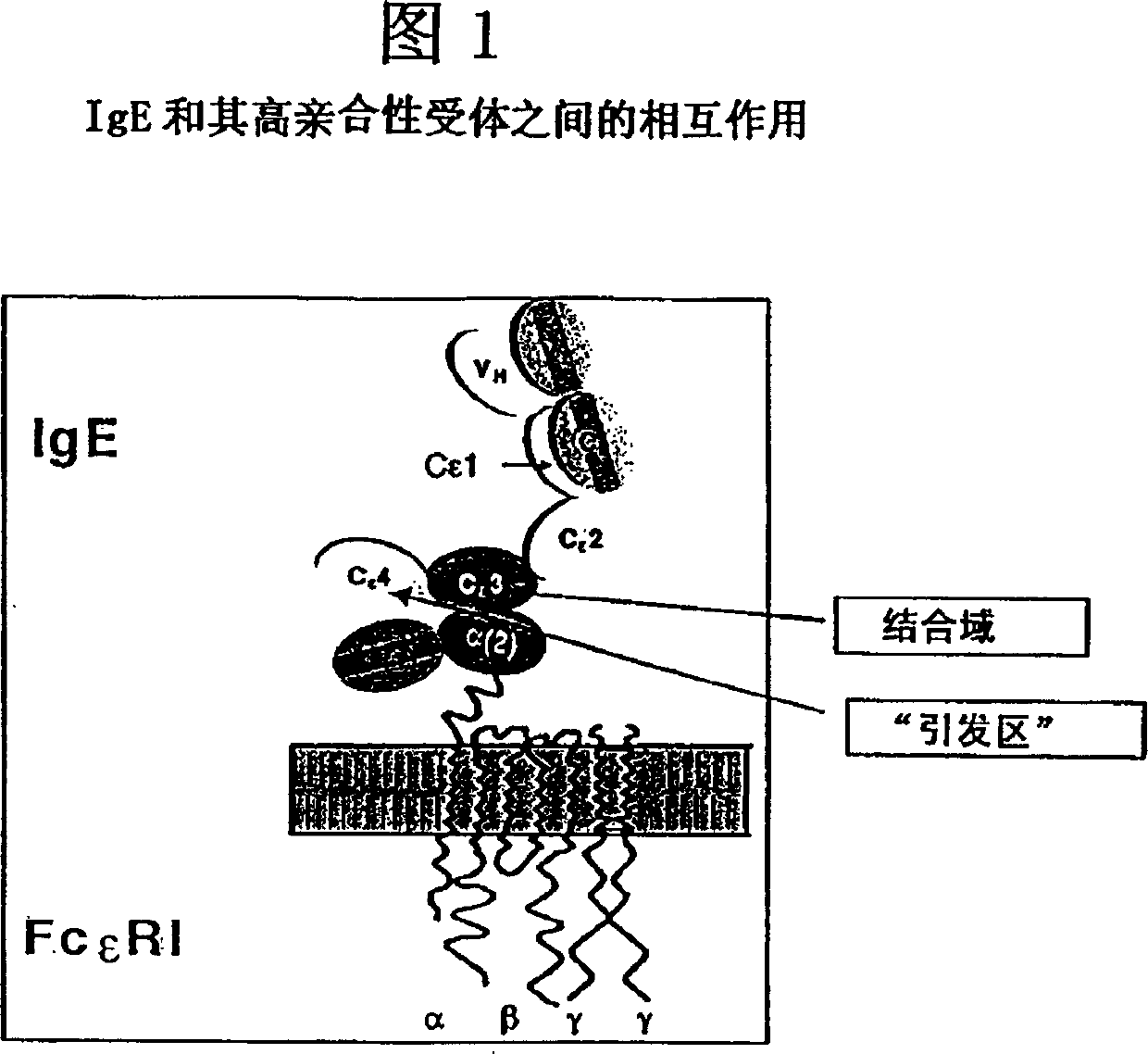
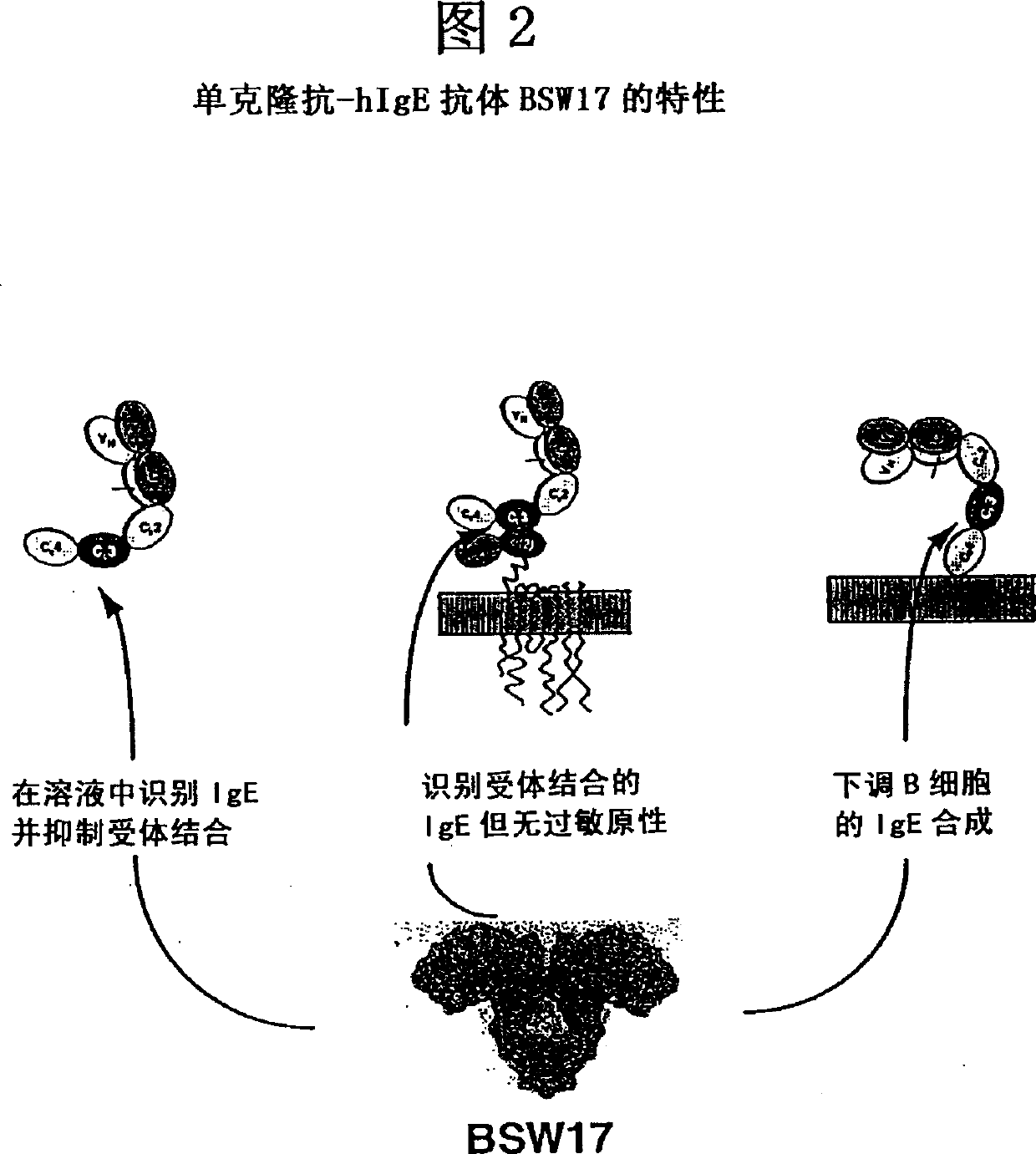
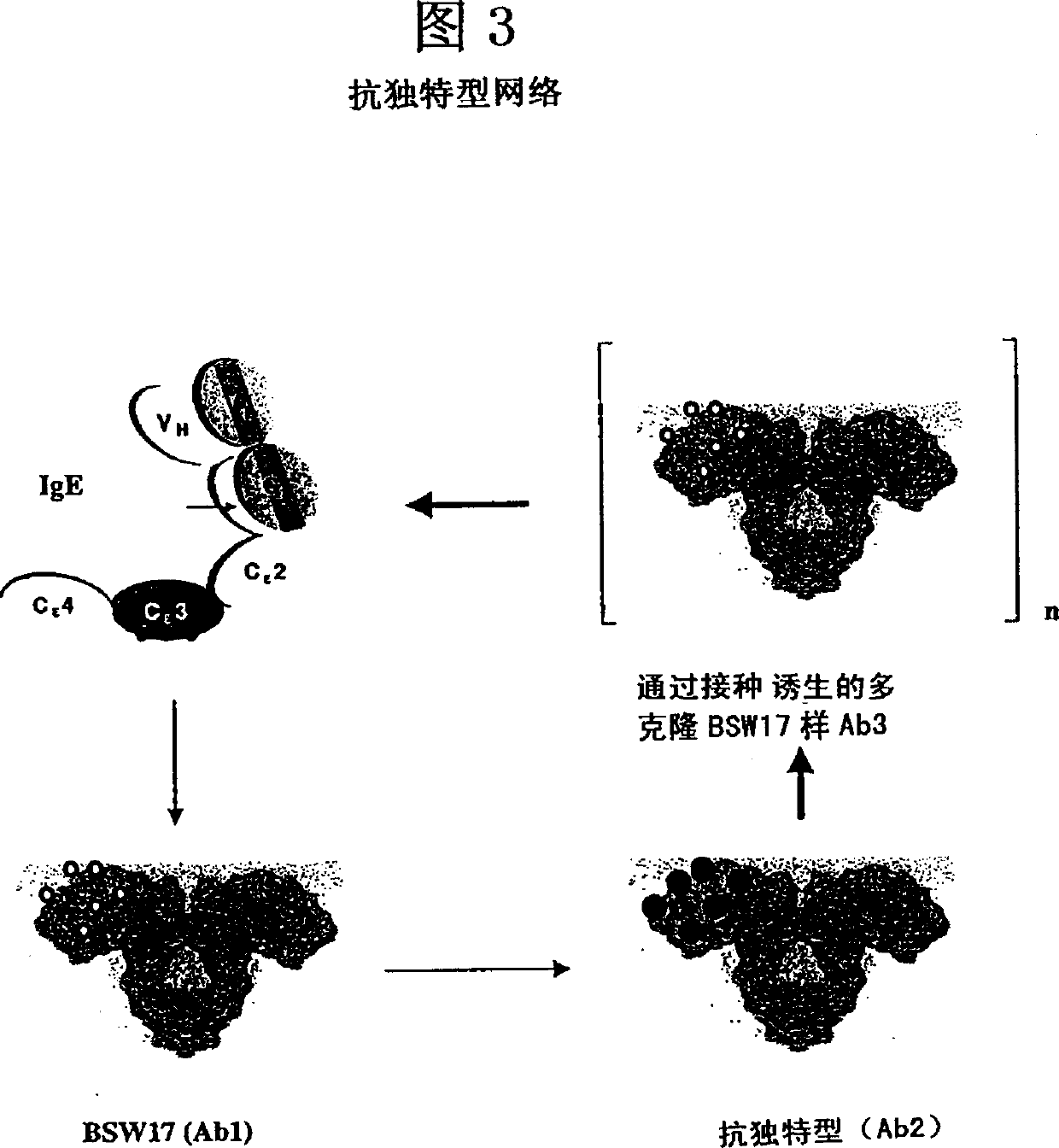
Anti-idiotypic antibodies against antibodies which inhibit the binding of immunoglobuline to its high affinity receptor
A receptor binding, anti-idiotypic technology, applied in immunoglobulin, anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1: Construction of phage display library
[0134] a) Source of lymphocytes
[0135] Mononuclear cells (NMC) were prepared from peripheral blood of two adult male donors. The first male adult atopic donor with clinical signs of allergy was boosted with an intramuscular injection of 0.5 ml of tetanus toxoid adsorbed on aluminum adjuvant (Te Anatoxal Bern, Swiss Serum and Vaccine Institute, Bern, Switzerland). Seven days later MNCs were isolated by gradient centrifugation using Ficoll (Pharmacia, Lymphoprep, Milwaukee, WI, USA). Then with 10 3 U / ml interleukin 2 (Sigma, St-Louis, MO, USA), 50 μg / ml Pansorbin cells (Staphylococcus aureus Cowan strain 1, Calbiochem, La Jolla, California, USA) and 1:1000 dilution with RPMI-1640 medium Tetanus toxoid was cultured in RPMI-1640 (Seromed, Basel, Switzerland) medium for 3 days. Total RNA was prepared from these cells using the phenol-chloroform guanidine isothiocyanate procedure (Chomczynsi, P. and Sacci, N., Aha...
Embodiment 2
[0164] Example 2: Screening of recombinant BSW17-specific antibody fragments (BSW17- mock antibody)
[0165] BSW17-specific phage selection was performed by four rounds of panning. In each round, two pre-adsorptions were performed with anti-IgE-mAb Le27 prior to adsorption of anti-IgE mAb BSW17. Pre-adsorption was performed as follows: two immunotubes (Maxisorp, Nunc) were coated with 4ml Le27 (20 μg / ml) overnight at 4°C and then blocked with 4ml PBS / 2% skim milk for 2 hours at 37°C. The first tube contains 2 x 10 in 4ml 12 The cfu blocking solution of each phage library (BS, LD2 and CT) was incubated with shaking at room temperature for 30 minutes. The phages were then transferred to a second tube and the procedure repeated. After the second pre-absorption, non-Le27-specific phages were added to tubes coated with 4 ml BSW17 (20 μg / ml) and blocked with PBS / 2% skim milk as above. After incubation at room temperature for 2 hours with shaking. The tubes were washed succe...
Embodiment 3
[0169] Example 3: Nucleotide sequence of recombinant BSW17-mimetic antibody
[0170] Plasmid DNA was prepared from selected phage clones using the Nucleotrap kit (Machery-Nagel, Duren, Germany). And use the PRISM Ready Reactin DyeDeoxy Terminator CycleSequencing Kit (Applied Biosystems, Germany) kit to perform nucleic acid sequence determination on the ABI 373A sequencing system.
[0171] Primers used for heavy chain sequencing were:
[0172] CHγ1(5′-CGCTGTGCCCCCAGAGGT-3′)( Seq.id.no.20 )and
[0173] pCH(5′-GGCCGCAAATTCTATTTCAAGG-3′)( Seq.id.no.21 ).
[0174] To obtain the light chain sequence, the following primers were used:
[0175] Cλ(5′-GAGACACACCAGTGTGGC-3′)( Seq.id.no.22 ),
[0176] CK(5′-CACAACAGAGGCAGTTCC-3′)( Seq.id.no.23 )and
[0177] pCL(5′-CTAAACTAGCTAGTCGCC-3′)( Seq.id.no,24 ).
[0178] Primers were synthesized by Microsynth (Balgach, Switzerland). From the DNA sequences of the selected different phage clones, two different amino acid sequences...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com