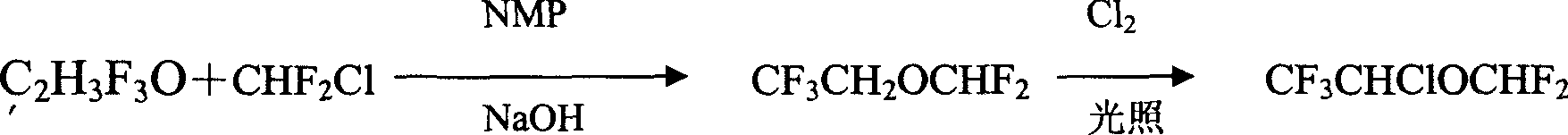Preparation method of isoflurane
A technology of isoflurane and trifluoroethanol, which is applied in the field of preparation of 1-chloro-2, organic compounds, can solve the problems of high impurity content in the final product, difficulty in ensuring the yield of isoflurane, and difficulty in achieving repeated rectification, etc. Achieve the effects of easy quality control, improved yield and stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
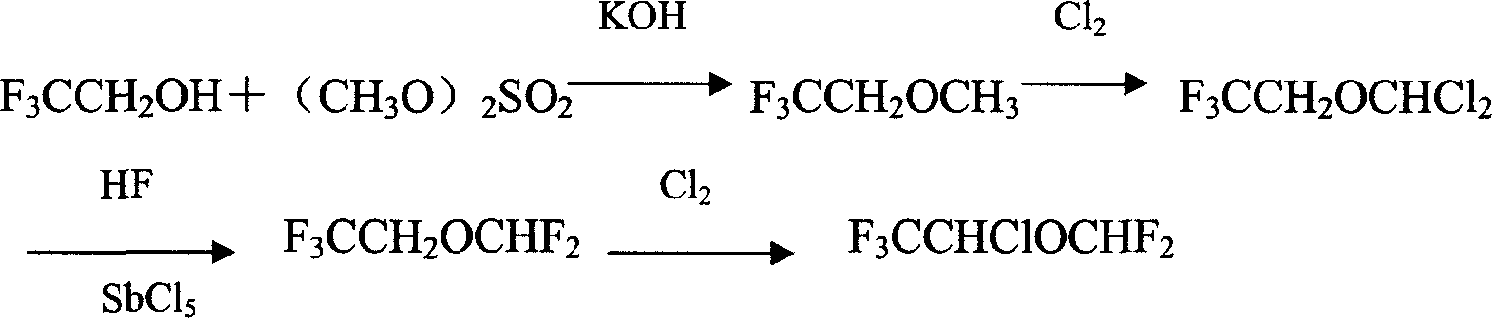
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of 2,2,2-trifluoroethyl difluoromethyl ether
[0020] Add 200g of TFE and 80g of NMP into the autoclave, close the autoclave, start stirring, add 173g of Freon-22 (F-22), then fill the autoclave with nitrogen, add 368g of 50% NaOH aqueous solution at 5°C under stirring, and control The temperature in the autoclave is between 36°C, after the addition, fill the autoclave with nitrogen to 18-20kg / cm 2 , stirred for 3 hours. Transfer the reaction solution in the still to the distillation still, heat and fractionate in a water bath with stirring, collect fractions below 30°C to obtain CF 3 CH 2 OCHF 2 180g, the residue continued to distill, reclaimed TFE60.5g, conversion rate was 60%, and yield was 86% (according to the TFE consumption calculation).
Embodiment 2
[0021] Embodiment 2: the preparation of isoflurane
[0022] Put 300g (2.0mol) 2,2,2-trifluoroethyl difluoromethyl ether into the chlorination tank, turn on the stirring and fluorescent lamp, and feed a small amount of chlorine gas. When the color of the chlorine gas in the tank disappears, use a gas flow meter to control The speed of passing chlorine gas makes it react completely without overflowing. The water bath controls the reaction temperature at 15-20° C., feeds 0.9 mol of chlorine gas, and then reacts at 15-20° C. for 30 minutes. The reaction liquid was distilled, and the fraction below 40°C was collected as the etherified product to obtain 150 g. Cool the residue to 5-10°C, slowly add sodium hydroxide solution dropwise under stirring, stir for 10 minutes after adding, let stand for 10 minutes, put the lower layer chloride solution into the storage tank, wash it with water three times, and remove the lower layer chloride solution The solution was put into a drying tan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com