Post-transition metal catalytic system for synthesizing branched polyethylene and its use
A late-transition metal and branched polyethylene technology, which is applied in the field of late-transition metal composite catalytic systems, achieves the effects of low price, simple polymerization process, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0029] Example 11 is the polymerization result obtained by adopting late transition metal catalyst ethylene homopolymerization
[0030] The specific description of embodiment is as follows:
Embodiment 1
[0032] (1) Synthesis of catalyst
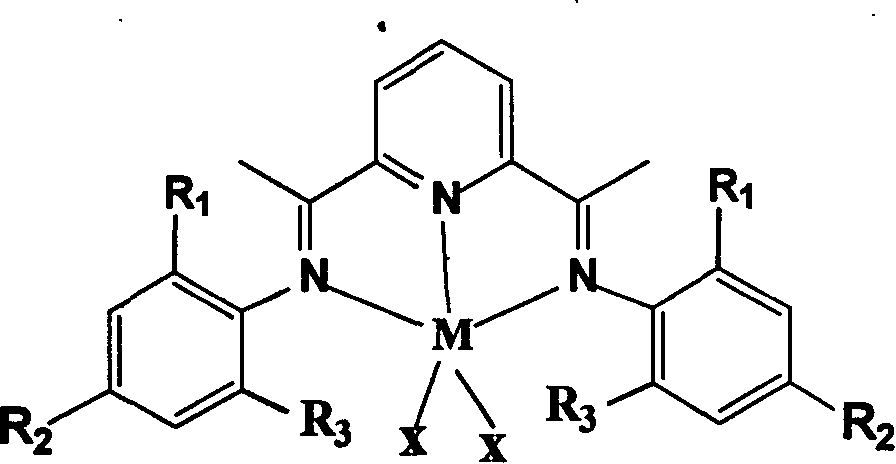
[0033]At room temperature, weigh two parts of 2,6-diacetylpyridine, 0.5g each, 2ml of 2-methylaniline and 4ml of 2,6-dimethylaniline, and mix the above-mentioned part of 2,6-diacetylpyridine with 2 -Methylaniline was added to a round-bottomed flask, another part of 2,6-diacetylpyridine and 2,6-dimethylaniline was added to another round-bottomed flask, and then (30ml) 1,2 -dichloroethane, finally add five drops of formic acid, reflux cooling at 50°C for 48h, evaporate the solvent, add methanol and freeze at low temperature to obtain pale yellow crystal ligands A and B. Weigh 0.3g each of ligands A and B, add them to two single-necked flasks protected by argon and contain 20ml THF respectively, add 50mg metal halides to each, and carry out the reaction at room temperature for 2h. After the reaction, add 20ml diethyl ether, Finally, it was filtered and vacuum-dried at room temperature for 2 hours to obtain the blue iron oligomerization catalyst...
Embodiment 2
[0038] (1) Synthesis of catalyst
[0039] At room temperature, weigh two portions of 2,6-diacetylpyridine, 0.5g each, 2-methyl-4-chloroaniline (3ml) and 2,6-dimethylaniline (4ml), and the above-mentioned portion of 2, Add 6-diacetylpyridine and 2-methyl-4-chloroaniline to one round bottom flask, and another part of 2,6-diacetylpyridine and 2,6-dimethylaniline to another round bottom flask In the flask, add (30ml) 1,2-dichloroethane respectively, and finally add five drops of formic acid each, reflux cooling at 50°C for 48h, evaporate the solvent, add methanol and freeze at low temperature to obtain light yellow crystal ligands C and D until. Weigh 0.3g each of ligands C and D, add them to two single-necked flasks containing 20ml THF protected by argon, add 45mg of metal halides to each, react at room temperature for 2h, and add 20ml diethyl ether after the reaction , and finally filtered and dried in vacuum at room temperature for 2 hours to obtain the blue iron oligomerizat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com