Method for synthesizing 4-methoxy styrene
A technology of methoxystyrene and methoxyacetophenone, which is applied in the field of synthesis of 4-methoxystyrene, can solve the problems of cumbersome preparation of raw material amides, unsuitability for industrial production, and many olefin by-products. The effect of cheap raw materials, high yield, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
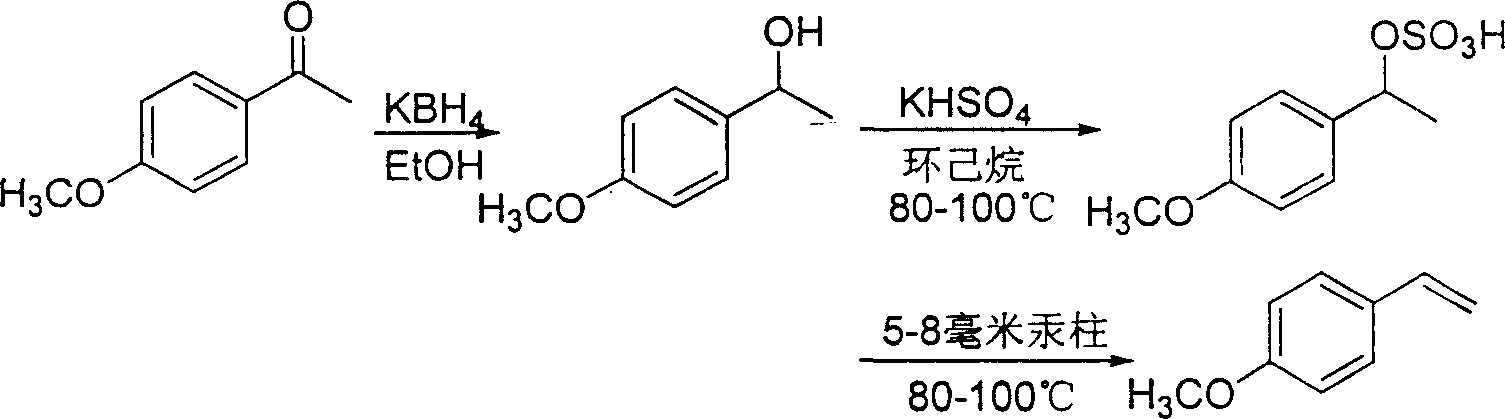
[0029] Embodiment one: now the concrete process step of the inventive method is described as follows:
[0030] a. Reduction reaction: Add 1 kilogram (6.659 moles) of 4-methoxyacetophenone in a 5-liter round-bottomed flask, 2 liters of industrial alcohol, stir, and after dissolving, add 120 grams of potassium borohydride (2.224 mol), while keeping the reaction temperature at 20°C-40°C, react for 6 hours. After the reaction, add an equal volume of water and stir well to hydrolyze excess potassium borohydride and borate complex. Then, 2 liters of ethyl acetate was added for extraction, and the layers were separated to obtain an organic layer. The aqueous layer was extracted twice with a small amount of ethyl acetate, and the organic layers were combined. Use anhydrous magnesium sulfate to dry, spin dry solvent with rotary evaporator again, obtain 0.993 kilograms of product 1-(4'-methoxyphenyl) ethanol, productive rate 98% (productive rate is based on raw material 4-methoxyphenyl...
Embodiment 2
[0033] Embodiment two: present embodiment is substantially the same as embodiment one, and difference is that in step a, the amount of potassium borohydride used is 72 grams (1.332 moles), then the reaction time of step a is extended to 12 hours, 0.801 kg of the product 1-(4'-methoxyphenyl)ethanol was obtained with a yield of 79%. The final product 4-methoxystyrene obtained was 0.617 kg, with a total yield of 69.0%.
Embodiment 3
[0034] Embodiment three: present embodiment is substantially the same as embodiment one, and difference is, in step a, the amount of potassium borohydride used is 180 grams (3.329 mole), then the reaction time of step a is shortened to 4 hours, 0.989 kg of the product 1-(4'-methoxyphenyl)ethanol was obtained with a yield of 98%. The final product 4-methoxystyrene obtained was 0.758 kg, with a total yield of 84.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com