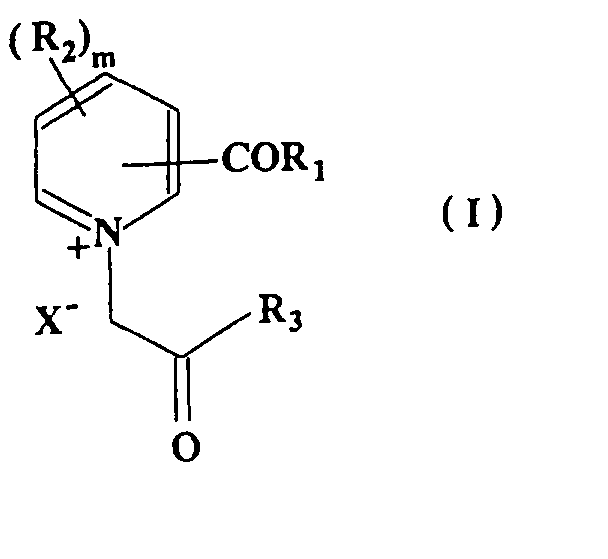
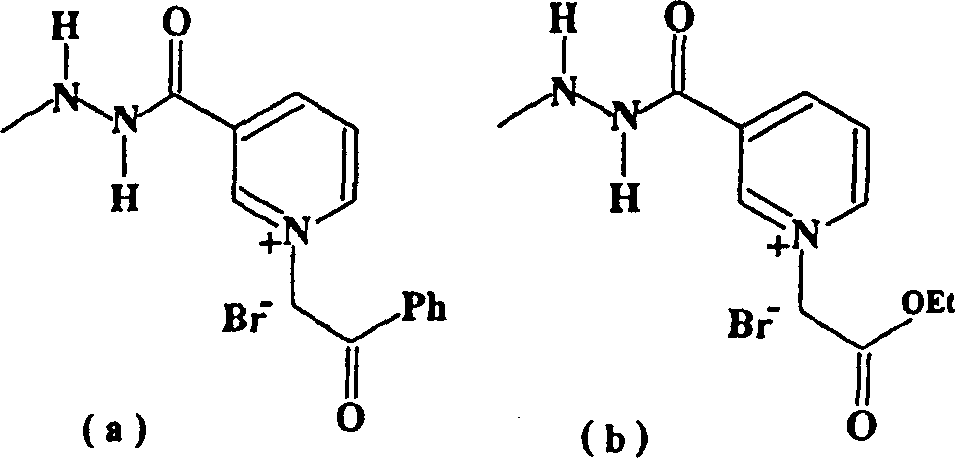
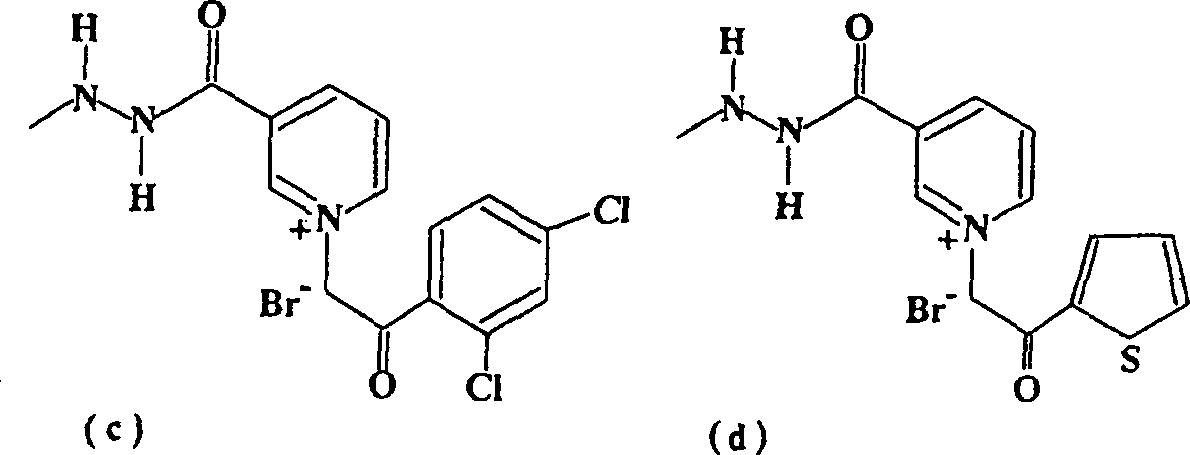
Pyridinum derivatives for management of aging-related and diabetic vascular complications, process for their prepn. and therepeutic uses thereof
A technology for diabetes and complications, applied in the field of new pyridinium series compounds, which can solve problems such as unclear mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 The following screening methods confirm the AGE destroyer activity:
[0056] Materials: Bovine Serum Albumin (Fraction V) (BSA) Ribose, Analytical Grade Phosphate Buffered Saline (PBS)
[0057] Equipment: Microplate ELISA Reader-Spectramax Plus (Molecular Devices, USA) Microplate Washer (Bio-Tec Instruments, USA) pH Meter
[0058] method:
[0059] 160 mg / ml of protein bovine serum albumin (BSA) and 1.6M glucose were dissolved in phosphate buffered saline (PBS). Add 0.02% sodium azide as a preservative. The solution was sterile-filtered with a 0.02 μm filter membrane and aged at 37° C. for 16 weeks. After 16 weeks, the solution was dialyzed against PBS, aliquoted and stored at -20°C.
[0060] In order to measure the AGE destroying activity, 10 μg / ml and 100 μg / ml AGE-BSA of 16 weeks were co-cultured with different concentrations of test compounds at 37°C for 24 hours, and the AGE destroying activity was measured by ELISA.
[0061] ELISA was performed as fo...
Embodiment 2
[0081] Example 2 Preparation of N,N'-bis[3-carbonyl-1-(2-phenyl-2-oxyethyl)-3-pyridinium]hydrazine dibromide (compound 1):
[0082] Add a solution of phenacyl bromide (1.99g, 0.01mol) in isopropanol (10ml) to boiling N,N'-bis-(nicotinoyl)hydrazine (1.21g, 0.005mol) in methanol (20ml) , and the reaction mixture was refluxed for 6 hours. The reaction mixture was concentrated in vacuo to about 10 ml and filtered. The resulting residue was washed with hot ethyl acetate, and the isolated solid was crushed. It was recrystallized from a mixture of methanol:ethyl acetate (3:1, 20ml) to obtain a pale yellow solid. Yield: 60% m.p.: 260-262°C (decomposition) IR (KBr, cm -1 ):1696 and 1680 1 H NMR (DMSOd 6 ,400MHz)δ:11.65(2H,s),9.56(2H,s),9.21-9.16(4H,m),8.49-8.45(2H,m),8.08-8.05(4H,d),7.81-7.77( 2H,m),7.68-7.64(4H,m),6.58(4H,s) mass spectrum (m / z):479,480
[0083] According to the above method, the corresponding pyridine derivative is reacted with a suitable reagent, and the follo...
Embodiment 4
[0084] Example 4 N,N'-bis[3-carbonyl-1-(2-(2,4-dichlorophenyl)-2-oxyethyl)pyridinium]hydrazine dibromide (compound 3): Yield : 24% m.p.: 225-227 ℃ (decomposition) IR (KBr, cm -1):1702,1666 1 H NMR (DMSOd 6 ,400MHz)δ:11.69(2H,s),9.58(2H,bs),9.20-9.18(4H,m),8.49-8.47(2H,m),8.17-8.15(2H,d),7.92(2H, bs), 7.78-7.76 (2H, d), 6.50 (4H, s) mass spectrum (m / z): 615, 617, 618, 620. Example 5 brominated 1-(2-ethoxy-2-oxyethyl)-3- (2-(2-pyridyl)hydrazinocarbonyl)pyridinium (compound 4): Yield: 16% m.p.: 210-212°C IR (KBr, cm -1 ): 3140, 3005, 1732 and 1690 1 H NMR (DMSOd 6 ,400MHz) δ: 9.63(1H,s),9.27(2H,d),8.49-8.45(1H,m),8.13-8.07(2H,m),7.32-7.30(1H,m),7.12-7.11(1H ,m),5.77(2H,s),4.23(2H,q),1.25(3H,t) mass spectrum (m / z): 301,302
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com