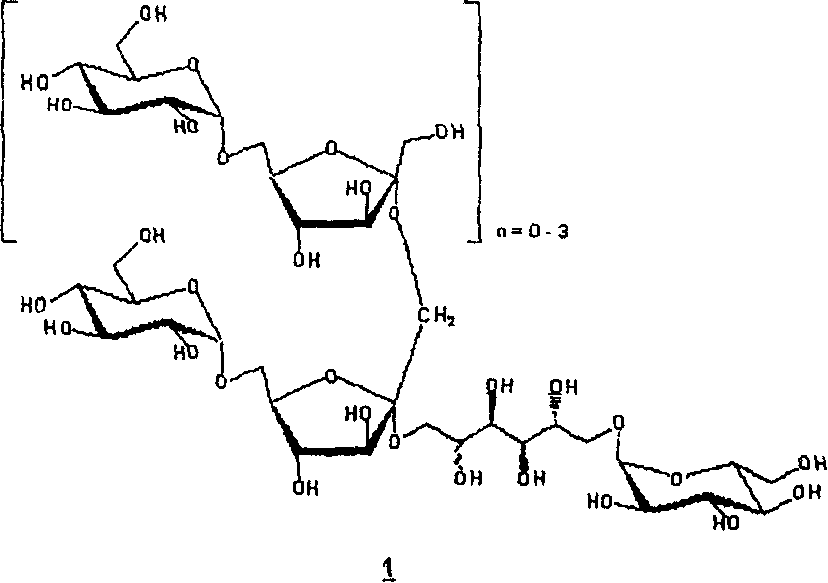
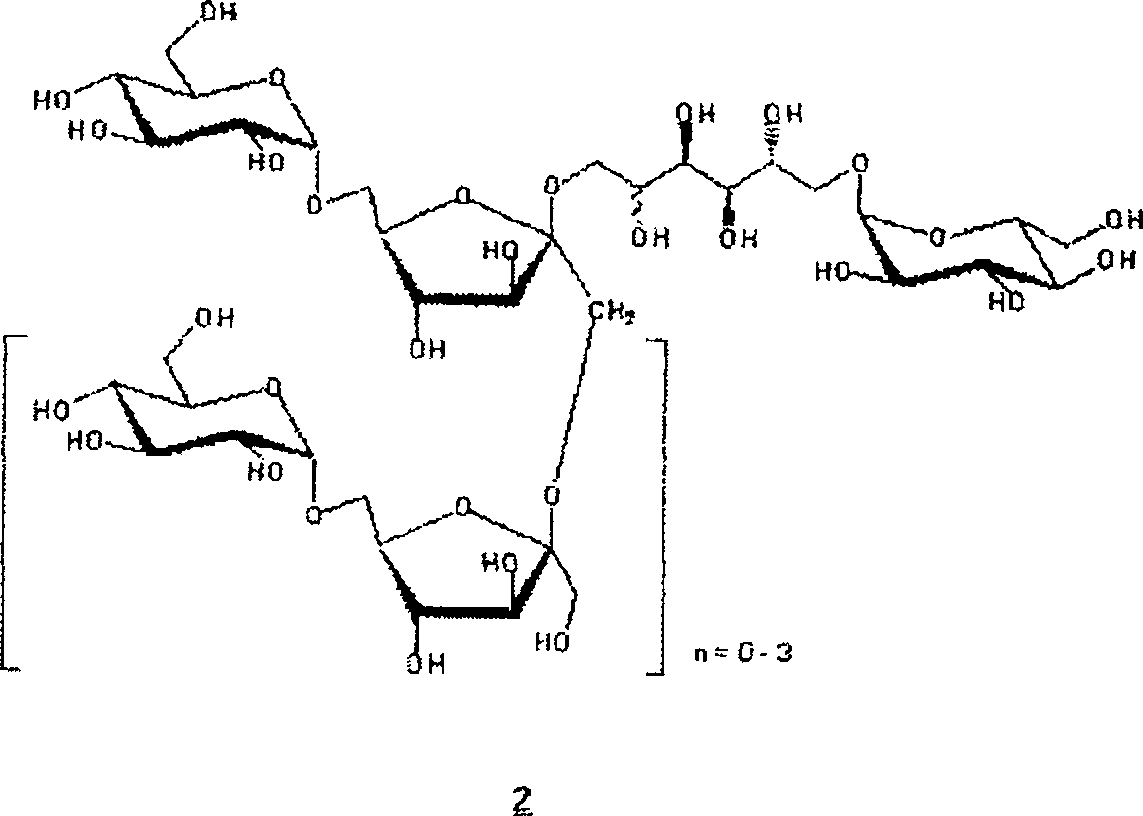
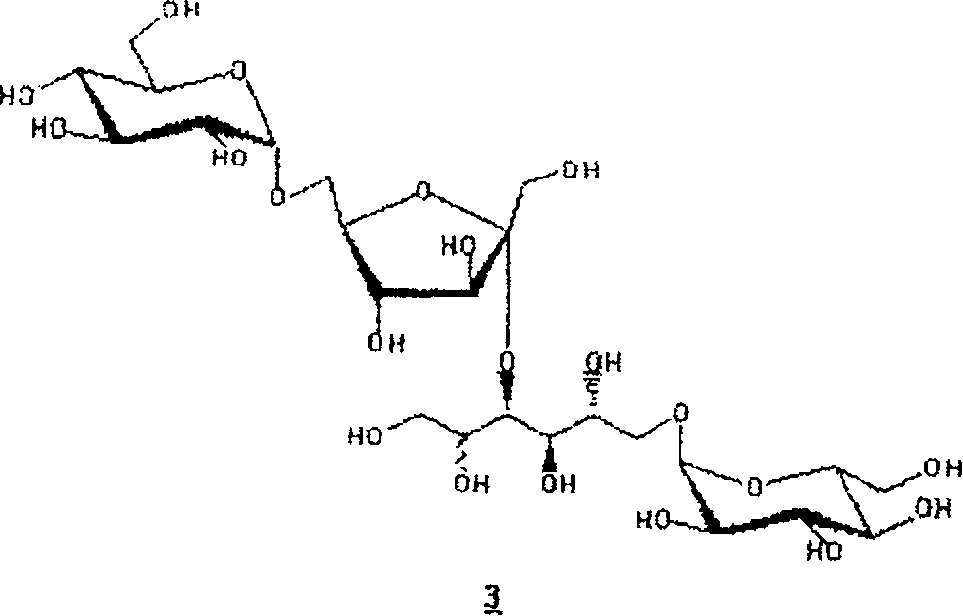
New hydrogenated condensed palatinose products
A palatinose and condensation technology, applied in oligosaccharides, drug combinations, organic chemistry, etc., can solve the problem of unstable resistant starch and achieve the effect of reducing reactivity and increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Preparation of Condensed Palatinose
[0129] 300 g of palatinose crystals and 90 g of water were added into a stainless steel container, stirred at 105° C. to dissolve, and then citric acid (0.02%, based on palatinose) was added. Condensate at 135°C under vacuum conditions, and keep the reaction for 30 minutes. The reaction product was dissolved in deionized water after cooling. The resulting solution was passed through the H + type cation exchange resin and OH - Type anion exchange resin for ion exchange to be purified. The following fractions were obtained by gel permeation chromatography:
[0130] The degree of polymerization ranges from 1 to 2%
[0131] The degree of polymerization ranges from 2 to 48%
[0132]The degree of polymerization ranges from 4 to 28%
[0133] The degree of polymerization ranges from 6 to 12%
[0134] The degree of polymerization ranges from 8 to 5%
[0135] The degree of polymerization ranges from 10 to 5%
[0136] The components ...
Embodiment 2
[0139] a) Hydrogenation of condensed palatinose
[0140] Get 500 milliliters of the reaction solution of 30% concentration obtained in Example 1, which contains 50% condensed palatinan, 2% monosaccharide and 40% isomaltulose, and the solution is adjusted under stirring with 1N NaOH pH to 7.8. The hydrogenation was carried out using nickel (wet weight 200 g) as a catalyst in the presence of hydrogen (150 bar) with stirring at 70°C.
[0141] Samples were taken at 0, 1, 2, 3 and 4 hours, and their isomaltulose content and 1,6-GPS (6-O-α-D-glucopyranosyl (1→6)- D-sorbitol) and 1,1-GPM (1-O-α-D-glucopyranosyl (1→1)-D-mannitol). When the quantitative free isomaltulose is converted into 1,1-GPM and 1,6-GPS, the hydrogenation reaction is ended.
[0142] Hour
[0143] After 4 hours of reaction, the isomaltulose contained in the condensed palatinose solution (see Example 1) was completely hydrogenated to 1,6-GPS and 1,1-GPM. The solution obtained after separating the ca...
Embodiment 3
[0150] Labeling of Hydrocondensed Palatinose
[0151] The hydrogenated condensation palatinose product separated in Example 2 was partially hydrolyzed with HCl according to the following conditions:
[0152] Take 0.9 ml of 1% hydrogenated palatinose, mix with 0.1 ml of 1M HCl, and react at 47° C. for no more than 8 hours. Samples were taken at 0, 1, 2, 4, 6 and 8 hours and analyzed by the HPAEC method.
[0153] Hour
mg / L
0
1
2
4
6
8
1,6-GPS
0
12.8
13.6
14.1
14.2
14.4
1,1-GPM
0
13.3
14.1
14.7
15.3
15.4
0
50.0
54.5
57.0
57.3
57.9
Total
0
76.1
82.2
85.5
86.8
87.7
Isomaltulose / 1,6-GPS,
1, 1-GPM ratio
0
2∶1
2∶1
2∶1
2∶1
2∶1
[0154] Mild hydrolysis condition control makes th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com