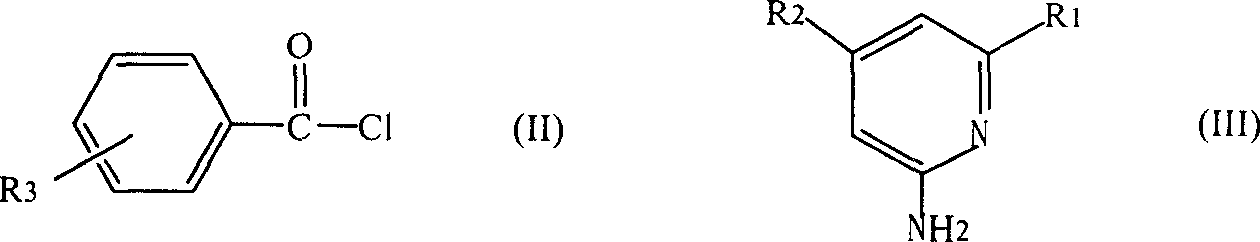Olefine oligomerization catalyst, and its preparation method and use
A catalyst and olefin technology, applied in the field of olefin oligomerization, can solve the problem of low oligomerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The preparation method of the catalyst provided by the present invention includes the following steps:
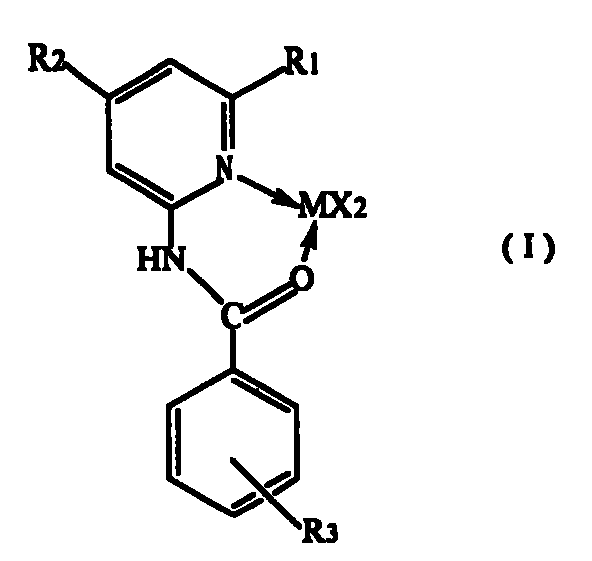
[0015] (1) The benzoyl chloride derivative represented by formula (II) is dissolved in tetrahydrofuran to make a solution, the 2-aminopyridine derivative represented by formula (III) is dissolved in pyridine to make a solution, and then the tetrahydrofuran The solution is added to the pyridine solution, and the compound of formula (II) and formula (III) are fully reacted at a molar ratio of 1 to 1.1:1 at 20 to 100°C, then washed with water and the solvent is removed to obtain N-pyridylbenzamides Ligand compound, R in the formula (II) 3 Selected from hydrogen, nitro or -CF 3 , R in formula (III) 1 , R 2 Respectively selected from hydrogen or C 1 ~C 6 的alkyl;
[0016]
[0017] (2) In tetrahydrofuran medium, make N-pyridylbenzamide ligand and MX 2 Or MX 2 ·DME reacts at 20-100°C at a molar ratio of 1-1.2:1, the MX 2 Where M is selected from Ni or Pd, X is selected from ha...
example 1
[0027] The catalyst {N-[2-(6-methylpyridyl)] benzamide} nickel dibromide is prepared.
[0028] (1) Preparation of ligand N-[2-(6-methylpyridyl)]benzamide
[0029]Dissolve 1.41 g of benzoyl chloride (10 mmol) in tetrahydrofuran to make a 10 ml solution. Add this solution dropwise to 1.08 g of 2-amino-6-methylpyridine (10 mmol) at 20°C. Ml of pyridine solution. Reaction at 25°C for 12 hours, 400 ml of deionized water was added to shake and wash and the reaction was terminated. The washed material was filtered, and the filtered solid was dried under reduced pressure for 4 hours to obtain 2.05 g of white solid, which is ligand a: N- [2-(6-Methylpyridinyl)]benzamide, yield 99% by mass. The analysis results of this ligand are as follows:
[0030] FT-IR (KBr disc, cm -1 ): 3192(m), 1677(s), 1577(s), 1459(s), 1304(s), 1129(m), 790(m), 719(m).
[0031] 1 HNMR(300MHz, CDCl 3 / ppm): 2.47(s, 3H, CH 3 ), 6.92-8.22 (m, 8H, ArH), 8.62 (s, 1H, NH).
[0032] Elemental analysis, measured (calculate...
example 2
[0040] Prepare ligand b according to the method of Example 1(1): N-[2-(4,6-dimethylpyridinyl)]benzamide, except that 2-amino-4,6-dimethylpyridine is used The reaction was performed instead of 2-amino-6-picoline to obtain 1.43 g of a white solid with a yield of 63% by mass. The analysis results of this ligand are as follows:
[0041] FT-IR (KBr disc, cm -1 ): 3183(w), 1676(s), 1614(m), 1567(s), 1421(s), 1282(s), 846(w), 706(s).
[0042] 1 HNMR(300MHz, CDCl 3 / ppm): 2.37(s, 3H, CH 3 ), 2.43(s, 3H, CH 3 ), 6.78-7.94 (m, 7H, ArH), 8.05 (s, 1H, NH).
[0043] Elemental analysis, measured (calculated) value, mass %: C, 74.43 (74.31); H, 6.24 (6.24); N, 12.17 (12.38).
[0044] The catalyst was prepared according to the method of Example 1(3), except that 1.1 mmol of ligand b was used for the reaction to obtain 0.3 g of orange-red powder, which was catalyst G: {N-[2-(4,6-dimethyl Pyridyl)] benzamide} nickel dibromide, the yield is 68% by mass. The analysis results are as follows:
[0045] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com