Method for modulating FXR receptor activity
A technology of receptors and ligands, applied in the direction of organic active ingredients, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as elusive methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
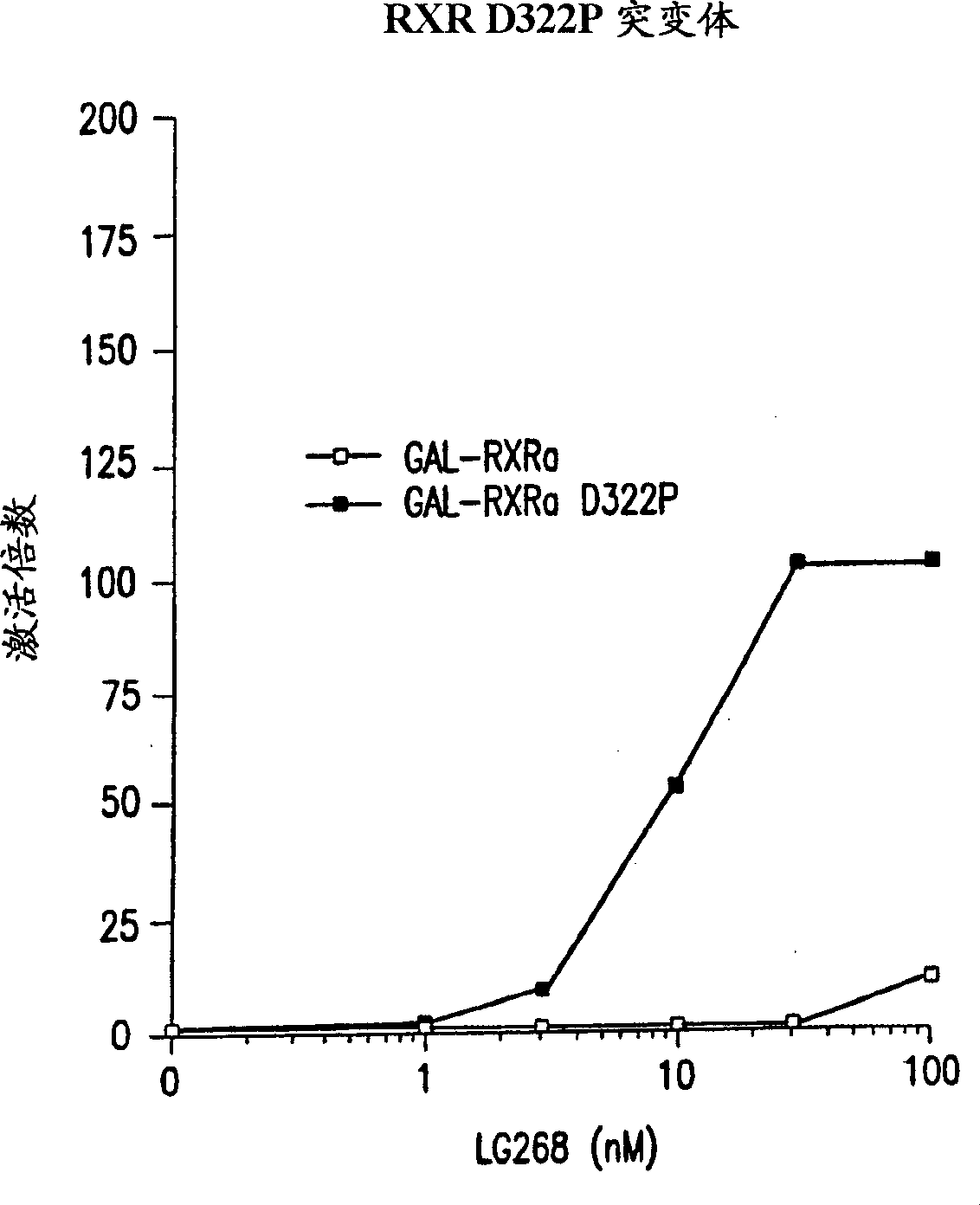
[0117] Because it is known that FXR binds to its response element in the form of a heterodimer combined with RXR, and because the heterodimer can be activated by the ligand of RXR; therefore, a mutant RXRα protein (RXR m ; also referred to as D322P in the accompanying drawings), the mutant RXRα protein contains a point mutation in the ligand-binding domain of RXR (Asp 322 →Pro). FXR-RXR m The use of heterodimers allows the unambiguous identification of modulators of FXR activity among test compounds.
[0118] Therefore, a reporter plasmid containing 4 copies of the GAL4 response element UAS positioned upstream of the firefly luciferase gene was constructed G ; the GAL4 response element UAS G It is also under the control of the herpes simplex virus thymidine kinase (TK) promoter. This reporter plasmid was co-transfected into African green monkey CV-1 cells with an expression vector (pCMX, which contains the cytomegalovirus CMV promoter upstream of the cloning site), which e...
Embodiment 2
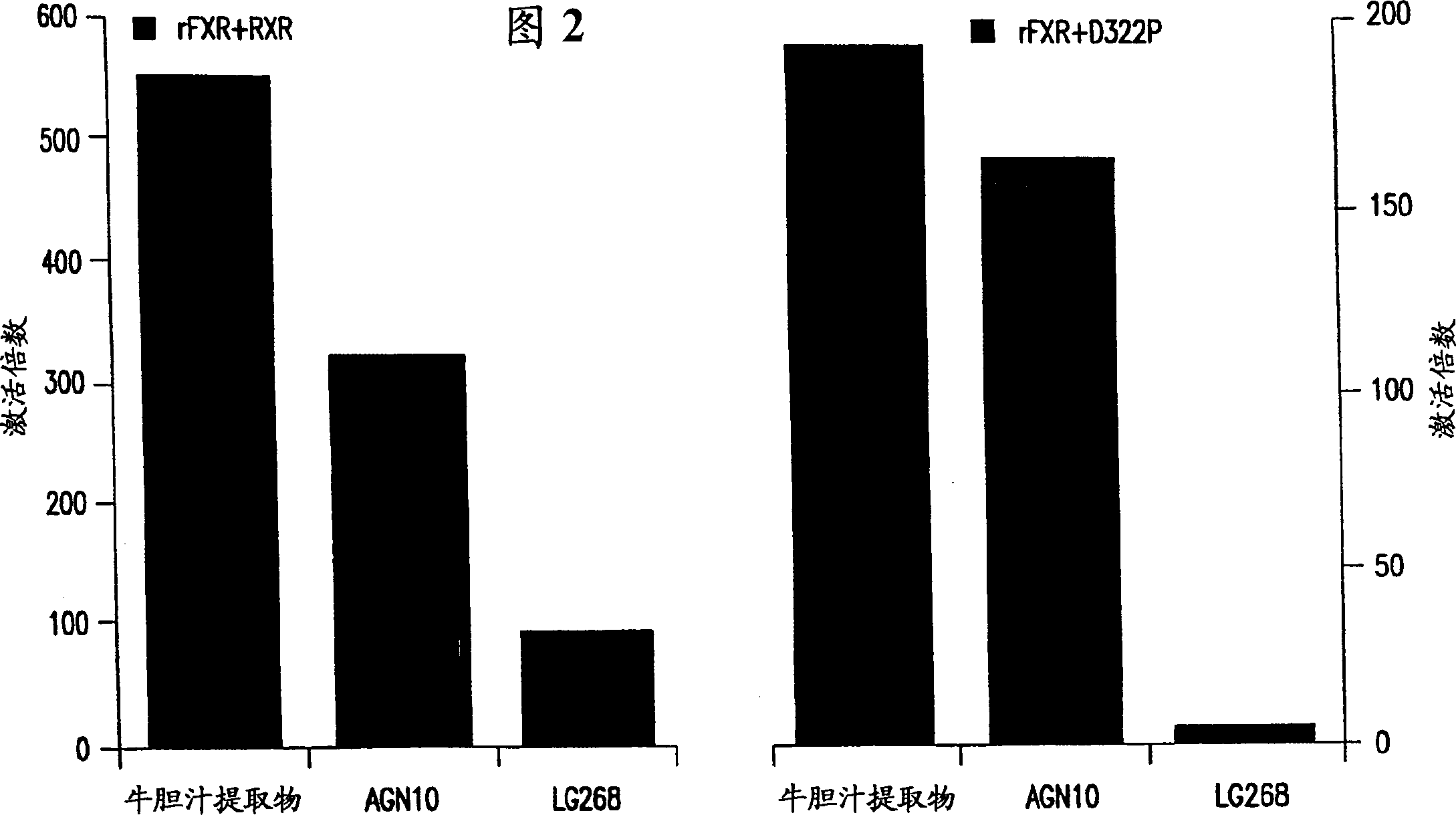
[0125] Because the transactivation activity of this FXR requires heterodimerization with RXR, although in ligand-dependent assays of FXR activity, RXR m Has a nonfunctional ligand binding domain; still do the following transactivation experiments to demonstrate RXR m retains its ability to potentiate FXR activity.
[0126] Recombinant full-length rat FXR and human RXR (or RXR) were cloned into the cloning site of plasmid pCMX using the transient transfection method generally described above. m ) were co-transfected into CV-1 cells. Additionally, the reporter plasmid was co-transfected with the expression plasmid described above.
[0127] By placing the cDNA coding sequence for firefly luciferase in frame immediately downstream of the herpes virus thymidine kinase promoter (located at nucleotide residues -105 to +51 of the thymidine kinase nucleotide sequence), to construct the luciferase reporter plasmid TK-Luc (described in Heyman et al., Cell 68:397 (1992), which is hereb...
Embodiment 3
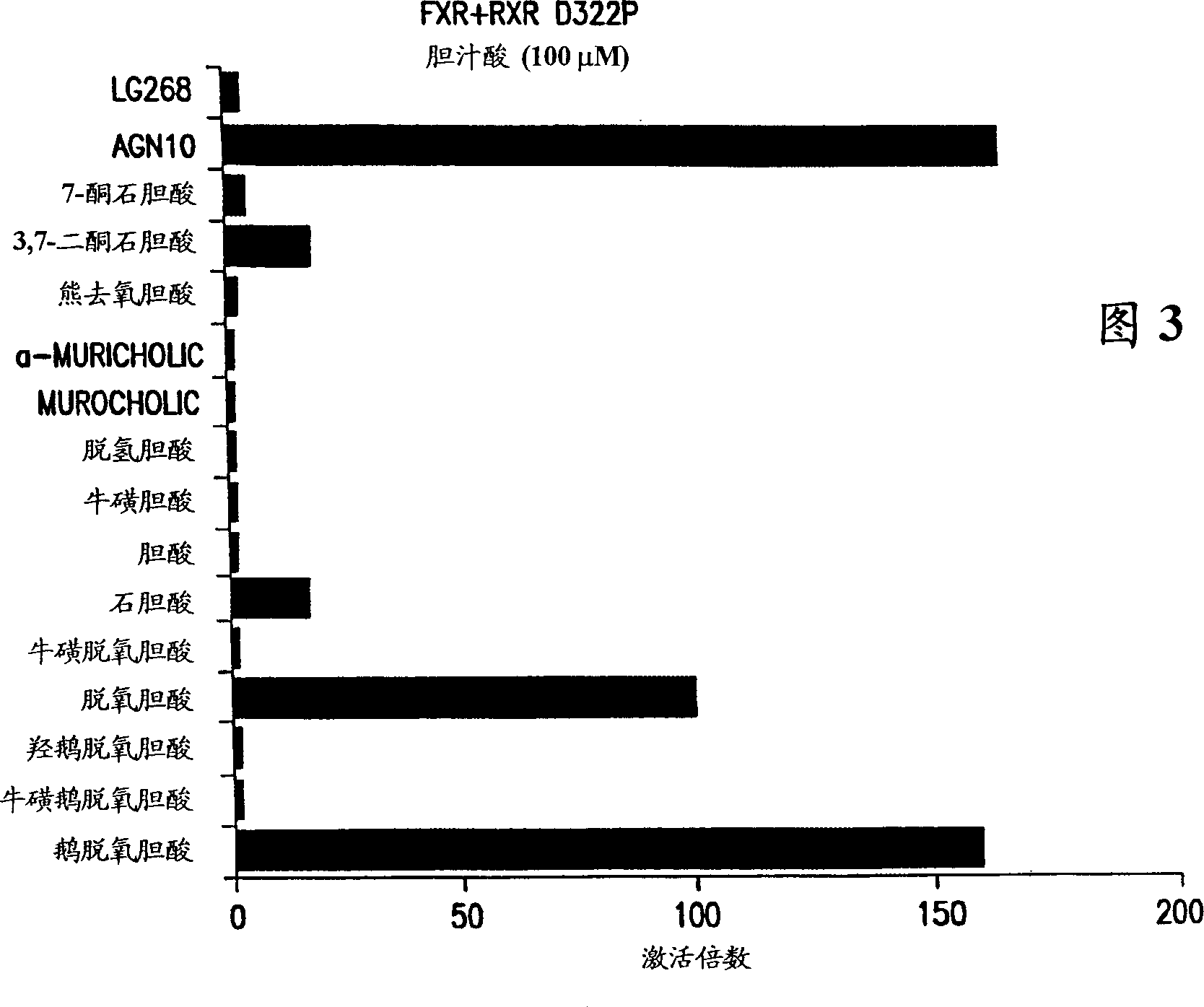
[0135] Using the FXR-RXR used in Example 2 m A co-transfection method comparing the FXR agonist activity of AGN10 with the FXR activity of different bile acids; including deoxycholic acid (DCA) and chenodeoxycholic acid (CDCA), which are known to be naturally occurring FXR ligands ). See: eg, Wang et al., Molec. Cell 3:543-553 (May 1999), which is hereby incorporated by reference.
[0136] Encoded with full-length FXR and RXR m , and the luciferase reporter plasmid used in the last experiment above, to co-transfect CV-1 cells. The cells were then incubated for approximately 44 hours with one of the following reagents at a concentration of 100 nM; the following reagents were: LG268, AGN 10 (10 μM), 7-ketolithocholic acid, 3 , 7-diketolithocholic acid, ursodeoxycholic acid, a-murocholic acid, murocholic acid, dehydrocholic acid, taurocholic acid, cholic acid (CA), lithocholic acid (LCA), taurodeoxycholic acid taurodeoxycholic acid, deoxycholic acid (DCA), hydroxychenodeoxych...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com