PCSK9 antagonist
An antagonist, antagonism technology, applied in the field of monoclonal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Build the Fab of pcsk9
[0080] Expression and purification of FABs from unique PCSK9 ELISA-positive clones
[0081] Fab (F16-15) from ELISA positive clones as well as EsB (negative control) Fab were expressed in E. coli TG1F-cells induced by IPTG. Cultures were lysed, histidine-tagged Fab purified by immobilized metal ion affinity chromatography (IMAC), and protein exchanged into 25 mM HEPES pH 7.3 / 150 mM NaCl by centrifugal diafiltration. Proteins were analyzed by electrophoresis on Caliper Lab-Chip90 and conventional SDS-PAGE, and quantified by Bradford protein assay. Serial dilutions of the purified Fab protein were re-assayed by ELISA to confirm the activity of the purified Fab. Positive and negative controls were performed as described above. Purified Fab preparations were analyzed using the EXOPOLAR (cholesterol uptake) assay as described below.
Embodiment 2
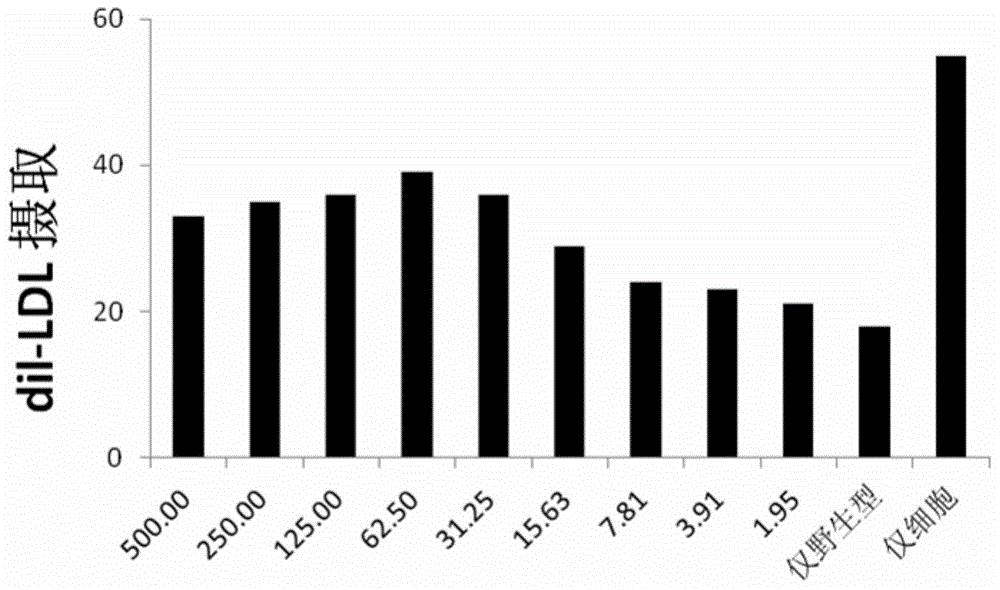
[0083] Epipolar assay: Effect of exogenous PCSK9 on cellular LDL uptake
[0084] On day 1, 30,000 cells / well were seeded in polyD-lysine-coated 96-well plates. On day 2, the medium was replaced with serum-free DMEM medium. On day 3, the medium was removed and the cells were washed with OptiMEM. Purified PCSK9 was added to 100 μl DMEM medium containing LPDS and dI-LDL. Plates were incubated at 37°C for 6.5 hours. Cells were washed rapidly in TBS containing 2 mg / ml BSA; then in TBS-BSA for 2 minutes; then washed twice in TBS (but rapidly). Cells were lysed in 100 μl RIPA buffer. Fluorescence in the plate was then detected with Ex520, Em580nm. The total cellular protein in each well was detected with the BCA protein assay and the fluorescence units (fluorescence units)
[0085] Normalized to total protein.
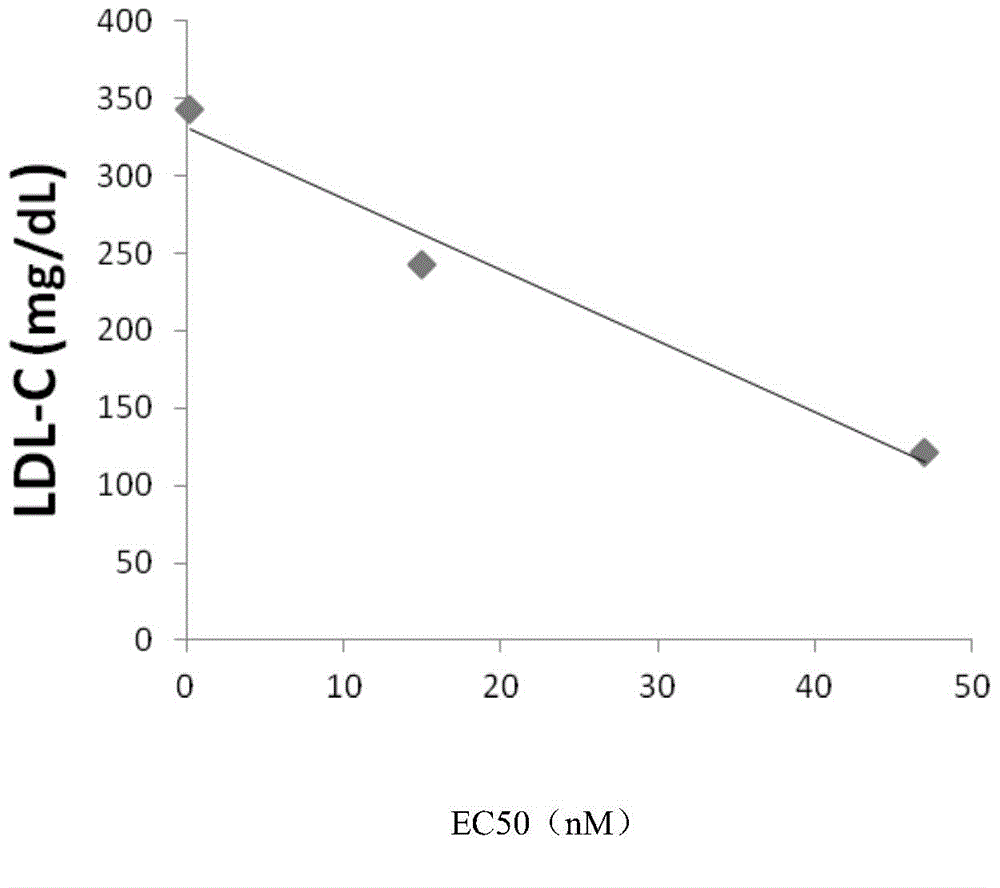
[0086] The epipolar assay is useful for identifying various effects on LDL uptake; see figure 2 , figure 2 Shows how the potency of PCSK9 mutants correlates with p...
Embodiment 3
[0093] Evaluation of Kinetics of Antibody Interaction with hPCSK9 Using Surface Plasmon Resonance ("SPR")
[0094] SPR detection was performed with a BiacoreTM (Pharmacia Biosensor AB, Uppsala, Sweden) 3000 system. Sensor chip CM5 and amine coupling kit for immobilization were purchased from BiacoreTM.
[0095] The antibody F16-15 was coupled to the FC2 channel of the CM5 chip by the amino coupling method in the Biacore3000 control software Wizard. HBS-EP was used as working buffer, 3.3mg / mL antibody F16-15 was diluted with pH4.26, 10mM NaAC to a final concentration of 11μg / mL. The surface of the chip was mixed with 0.2M EDC and 50mM NHS 1:1 and injected at a flow rate of 10μL / min for 7 minutes, then the antibody solution was injected, and injected with pH8.5, 1M ethanolamine for 7 minutes to block the activated chip surface. The final coupling amount of antibody F16-15 was 8916.1RU.
[0096] The hPCSK9 antigen was diluted to 0, 1.18, 1.68, 2.4, 3.4, 4.9, 7.0 and 10 nM with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com