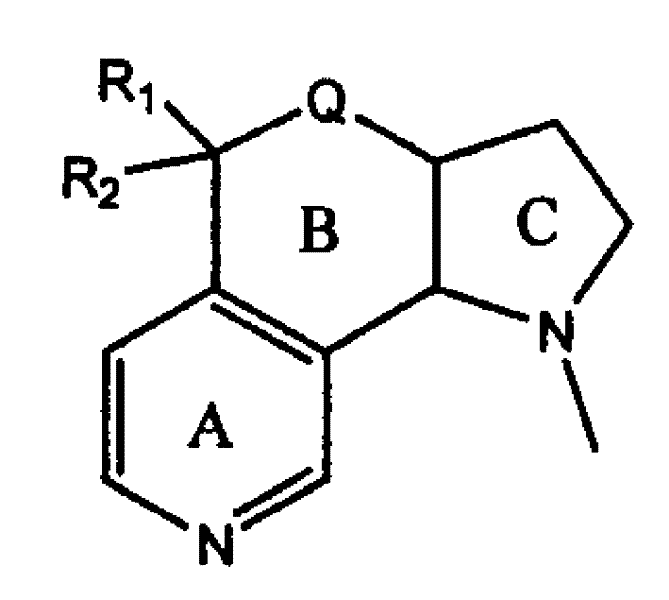
High-tension condensed ring nicotine analogue, preparation process and use thereof
A technology for nicotine and analogues, which is applied in the field of ternary condensed ring nicotine analogues, can solve problems such as limiting medicinal value, and achieve the effects of simple synthesis methods, high yields, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] n-Butyllithium (2.76 mmol) was added dropwise to a solution of compound 5 (2.3 mmol) in THF (25 mL) at -78°C. After stirring for 30 minutes, a solution of DMF (3.45 mmol) in THF (3 mL) was added dropwise at this temperature. Continue to stir the reaction for 1 hour, add saturated NaCO 3 The reaction was quenched by the solution, the organic solvent was spin-dried at room temperature, and the aqueous phase was washed with CH 2 Cl 2 Extraction, the combined organic phases were washed with brine, MgSO 4 After drying and separation by column chromatography, the intermediate alkene-substituted pyridine-3-carbaldehyde derivative (2) is obtained with a yield of 70-90%.
[0014] The result is as follows:
[0015] 1 H NMR (CDCl 3 , 300MHz) δ: 3.34(s, 3H), 4.63(d, J=6.6Hz, 1H), 4.79(d, J=6.6Hz, 1H), 5.22-5.42(m, 2H), 5.86-5.98(m , 2H), 7.64 (d, J=5Hz, 1H), 8.79 (d, J=5Hz, 1H), 9.01 (s, 1H), 10.31 (s, 1H).
[0016] MS (EI): 207 (M + ).
[0017] 1 H NMR (CDCl 3 , 30...
Embodiment 2
[0022] n-Butyllithium (2.76 mmol) was added dropwise to a solution of compound 5 (2.3 mmol) in ether (25 mL) at room temperature. After the reaction was stirred for 30 minutes, a solution of DMF (3.45 mmol) in diethyl ether (3 mL) was added dropwise at this temperature within 10 minutes. Continue to stir the reaction for 1 hour, add saturated NaCO 3 The reaction was quenched by the solution, the organic solvent was spin-dried at room temperature, and the aqueous phase was washed with CH 2 Cl 2 Extraction, the combined organic phases were washed with brine, MgSO 4 After drying and separation by column chromatography, the intermediate alkene-substituted pyridine-3-carbaldehyde derivative (2) is obtained with a yield of 70-90%.
[0023] 1 H NMR (CDCl 3 , 300MHz) δ: 1.67-1.80(m, 2H), 2.25-2.32(m, 2H), 3.34(s, 3H), 4.58(d, J=3.3Hz, 1H), 4.65(d, J=3.5Hz , 1H), 4.99-5.09(m, 2H), 5.49(t, J=6.2Hz, 1H), 5.77-5.93(m, 1H), 7.64(d, J=5.2Hz, 1H), 8.79(d, J=5.2Hz, 1H), 9.00(s, 1H), ...
Embodiment 3
[0027] Dissolve 4-allylpyridine-3-carbaldehyde derivatives (0.1mmol), N-methylglycine (0.3mmol) and dibutyltin dichloride (0.2mmol) in toluene, and react at 40°C for 5 hours or React at reflux temperature for 1 hour, and the product is separated by column chromatography to obtain a three-membered condensed ring nicotine analog with a yield of 55-80%.
[0028] The result is as follows:
[0029] 1 H NMR (CDCl 3 , 300MHz) δ: 2.19-2.27(m, 2H), 2.49(s, 3H), 3.00-3.13(m, 3H), 3.46(s, 3H), 3.87(d, J=7.8Hz, 1H), 4.80 -4.87(m, 2H), 5.12(d, J=4.5Hz, 1H), 7.32(d, J=5.2Hz, 1H), 8.52(d, J=5.2Hz, 1H), 8.68(s, 1H) .
[0030] 13 C NMR (CDCl 3 ,75MHz)δ:24.38,29.66,40.53,41.78,46.14,52.83,55.69,56.03,57.28,58.62,71.68,72.71,78.17,88.13,95.41,96.65,120.45,120.57,137.88,138.28,146.60,147.26,148.99 , 149.53, 151.70, 152.51.
[0031] MS (EI): 235 (M + +1).
[0032] Elemental analysis (C 13 h 18 N 2 o 2 ): Theoretical (%): C, 66.67; H, 7.69; N, 11.97. Measured (%): C, 66.39; H, 7.87;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com