HEV antigenic peptide and its method
A technology of antigens and antibodies, applied in chemical instruments and methods, botany equipment and methods, biochemical equipment and methods, etc., can solve problems such as difficulties in breeding viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] Serum—96 parts of serum described in the present invention are obtained from non-A, non-B, and non-C acute hepatitis patients who are onset or have been ill with the permission of Hong Kong Queen Margaret Hospital. Samples were collected from patients who were hospitalized or at various times after discharge. The extraction of the cloning HEV RNA of embodiment 1 HEV capsid gene
[0172] RNA was extracted from the liver juice of rhesus monkeys experimentally infected with HEV (Zhuang et al., 1992) using the QIAamp Virus RNA kit (QIAGEN GbmH, Hilden, Germany) according to the manufacturer's instructions. The purified RNA was mixed with two volumes of isopropanol (BDH Laboratory Supplies, England), and 1 / 10 volume of 3M sodium acetate (pH6.4) (Sigma, USA), and placed at -20°C for 1 hour. Afterwards, the mixture was centrifuged at 15,000 rpm for 15 minutes. The pellet was washed once with 70% ethanol (BDH Laboratory Supplies, UK), and then resuspended in reverse transcrip...
Embodiment 2
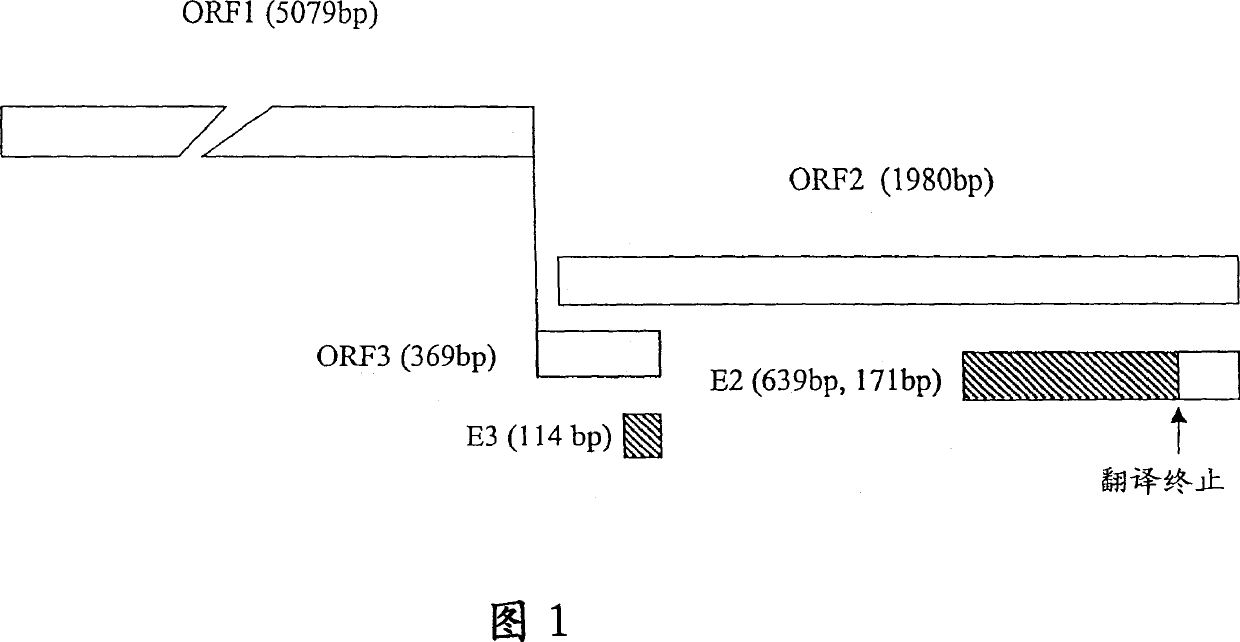
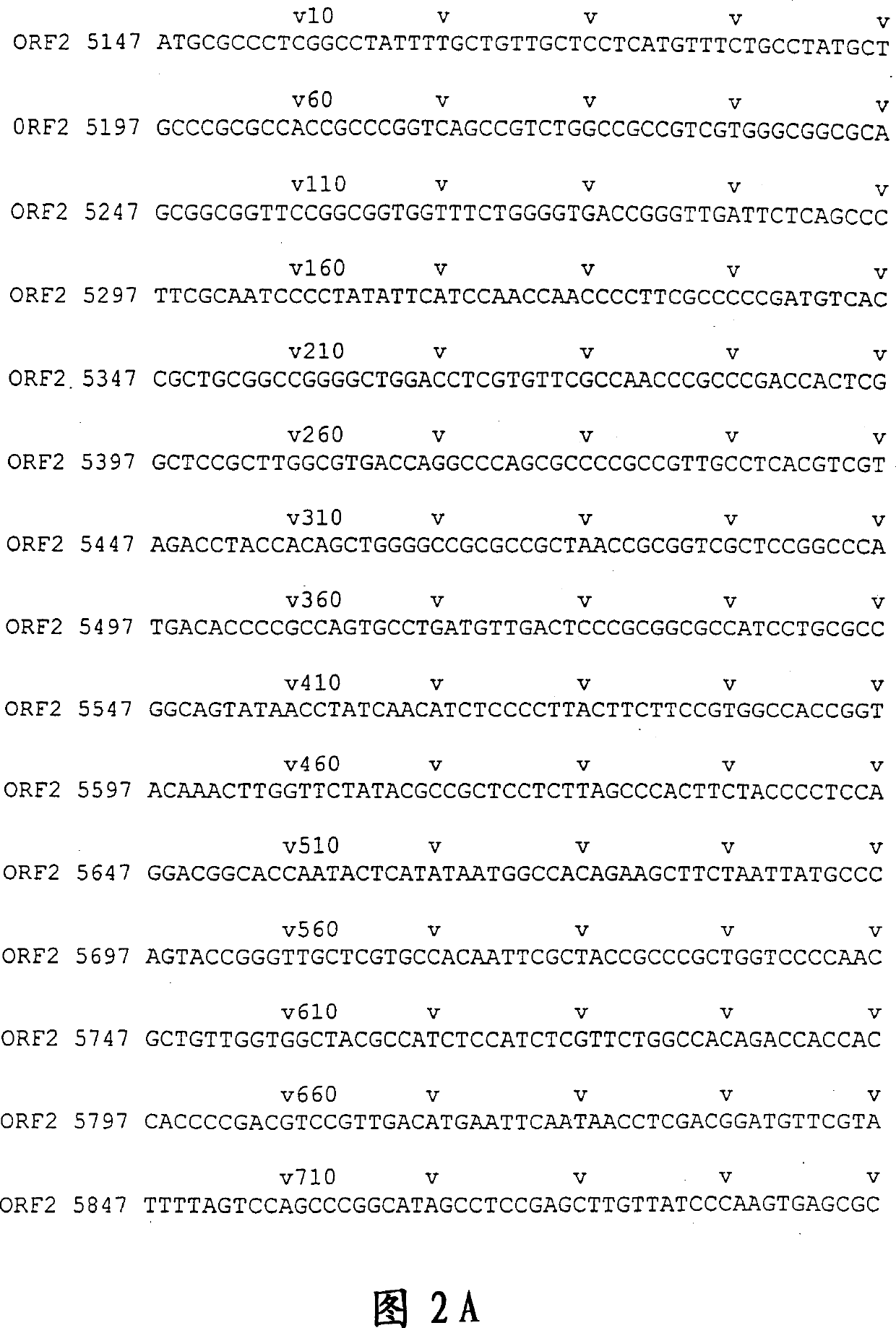
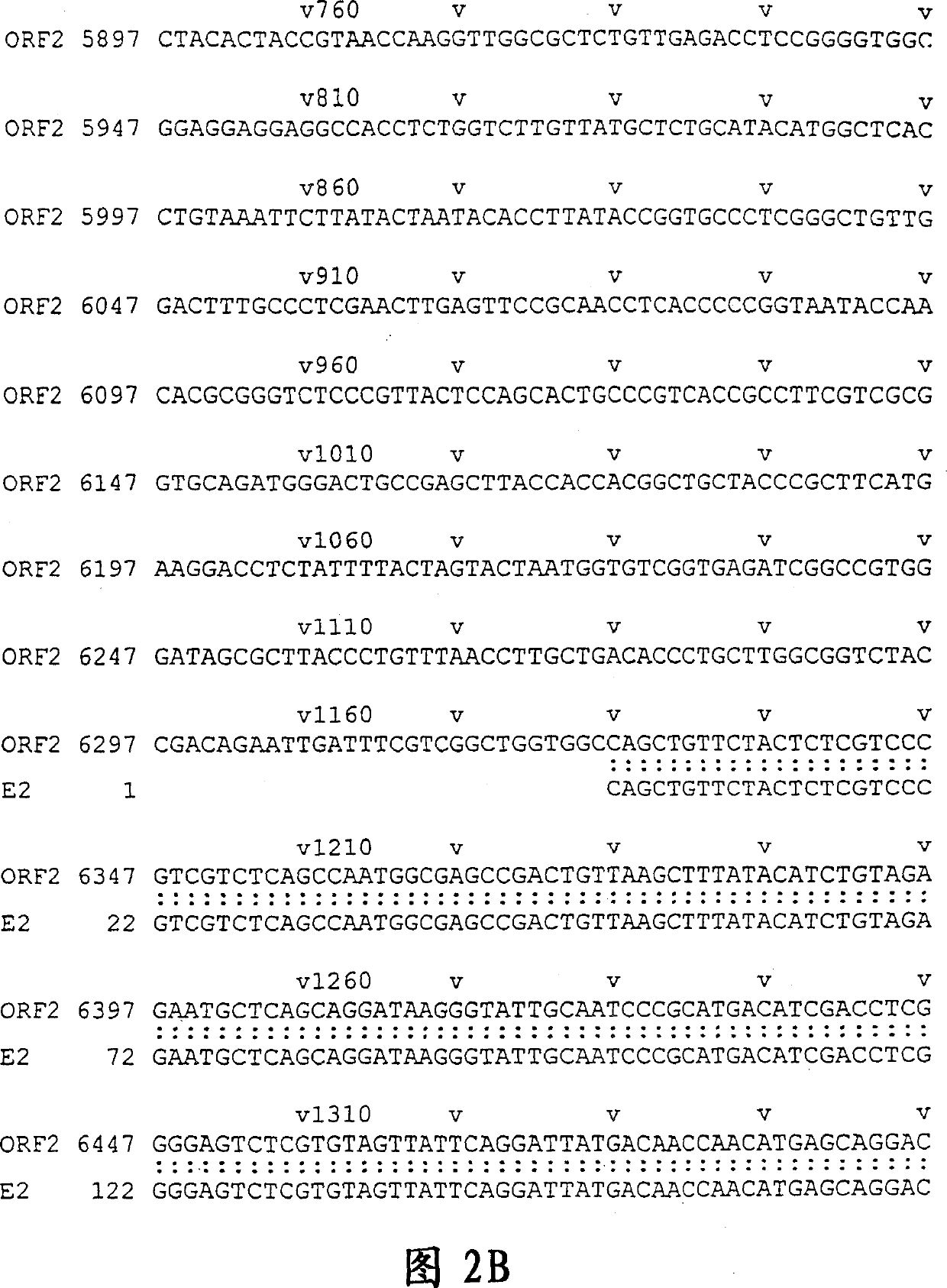
[0179] DNA sequencing was performed with ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit [PERKIN ELMER]. The results showed that the 811bp E2 sequence was located at positions 6326-7136, with a single base deletion at position 6957, presumably caused by PCR amplification errors. The resulting frameshift mutation caused premature termination of translation at a new stop codon at position 6968, resulting in a smaller-than-predicted 213 amino acid polypeptide with a molecular weight of 23 kD, rather than the originally expected 267 amino acids. The location of E2 and the associated fragments are shown in Figure 1. Example 2 Production of HEV Polypeptide and Expression of Purified GST Fusion Protein
[0180] The recombinant plasmid was transformed into BL21 E. coli. Pick a single clone in 2×YTA medium (peptone 16g / L; yeast extract 10g / L; NaCl 5g / L; penicillin 100ug / L). Inoculate 4ml of the overnight culture into 400ml of 2×YTA medium and culture at 28°C until OD600...
Embodiment 3
[0182] Thrombin [Pharmacia Biotech, USA] (5 ul of 1 U / ul solution) and 95 ul of PBS were added to 100 ul of GE2 bound to Glutathione Gel-4B and incubated at 22°C for 16 hours. The supernatant was collected by centrifugation, and the supernatants from the second substrate wash were pooled together. This protein cut out by thrombin is named pE2. Example 3 Identification of HEV polypeptide SDS PAGE (SDS polyacrylamide gel electrophoresis) analysis
[0183] 10% SDS polyacrylamide gels were prepared according to standard methods (Sambrook et al., 1989). 4ug polypeptide samples were loaded onto the gel, and electrophoresis was performed at 100 volts for 3 hours with a Minigel TwinG42 [Biometra, Germany] electrophoresis tank. The gel was stained with a Coomassie brilliant blue R250 mixture (45 ml methanol, 45 ml water and 10 ml glacial acetic acid). Western blot
[0184] After the SDS-PAGE, use the Mini-PROTEIN II Cell electrotransfer instrument [BIO-RAD] for one hour at 100 volt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com