Dihydrofuran heterocyclic compounds and synthesis process thereof
A technology of dihydrofuran and compounds, applied in the field of heterocyclic compounds and their synthesis, which can solve problems such as limited application range and severe reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
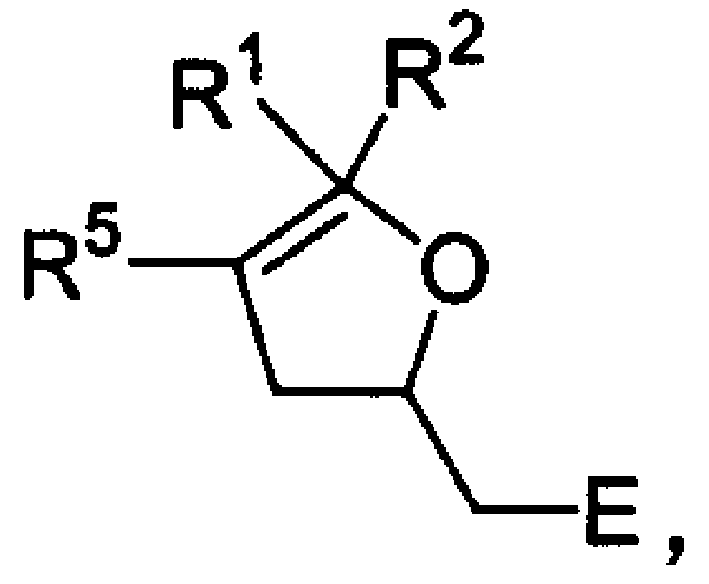
[0020] The following examples will help to understand the present invention, but do not limit the content of the present invention. Example 1: Reaction of an electron-poor allene with a carbon-oxygen double-center nucleophile in the presence of triphenylphosphine.
[0021] In toluene, add triphenylphosphine (0.2mmol) and corresponding carbon-oxygen double-center nucleophile (1mmol), heat up to 70-80°C to dissolve, then add electron-poor allene (1mmol) in toluene solution ( In the synthesis reaction of compound P2, P3, P4, P5, the toluene solution of electron-poor allene is slowly added dropwise to the reaction system with a syringe pump), and the reaction is continued until thin layer chromatography shows that the raw material disappears, the solvent is removed under reduced pressure, and the column Chromatography gave the corresponding dihydrofuran derivative. P1: (Reactants: ethyl 2,3-butadienoate, cyclohexanedione) IR (neat) ν2947, 1736, 1635, 1404, 1181cm -1 . 1 H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com