Recombinant eggs and gene cloning and expression vectors based on avian adenoviruses
An adenovirus, avian technology, applied in the field of molecular biology, can solve problems such as expensive, difficult and unpredictable basic manufacturing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Using the results of Swedish researchers describing the homology between the hexon genes of human Ad2 and Ad CELO (J.Virology-1982, v.42, N1, 306-310), we synthesized some primers, which It is the unique characteristic sequence of human Ad2 hexon gene. Primer specificity was determined by sequencing cloned fragments from Ad CELO and human Ad5. One of the primers was used to synthesize an Ad CELO fragment that hybridized to the human Ad2 hexon gene. The reverse primer is from human Ad2 genome position: 21164-21185bp
[0062]5'AGGAACCAGTCTTTGGTCATGT-3' SEQ ID NO: 27
[0063] This primer has been used for cDNA synthesis of the Ad CELO hexon gene. First-strand cDNA synthesis was performed with AMV reverse transcriptase. For second strand synthesis RNase H, DNA-pol 1 and T4 DNA-pol were used. The double-stranded cDNA (approximately 2500-3500 nucleotide base pairs) was washed from the agarose gel and cloned into pBluescript II SK(+) vector. Clones were selected by molec...
Embodiment 2
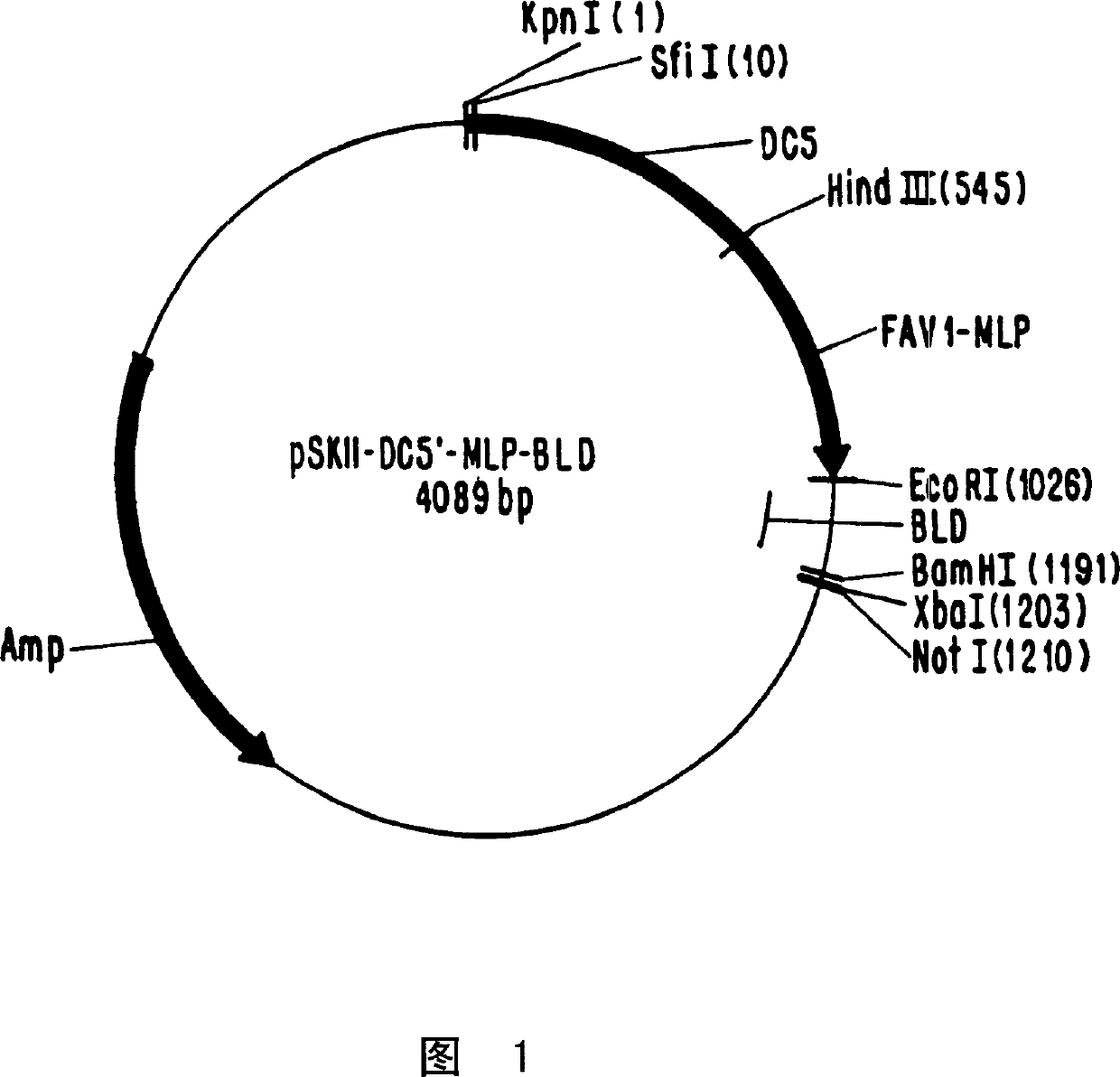
[0065] This example describes the generation of the plasmid pSKII-DC5'-MLP-2bLd, which is depicted in Figure 1 . Plasmid pSKII-DC5'-MLP-2bld was prepared by directional cloning in 3 sequential steps.
[0066] 1. Cloning of the 5' end of the FAV1 genome
[0067] First, by using oligonucleotide primers and polymerase chain reaction (PCR), up to 538 base pairs (bp) of the left end of the Ad CELO genome (located between 0 and 538 bp DNA sequence of the avian adenovirus CELO genome Between) were amplified and isolated. The primers used were:
[0068] 5'-CAAGTGGTACCGGCCAAATTGGCCGATGATGTATAATAACCTCA-3' [SEQ ID
[0069] NO: 1]
[0070] 5'-CAACCAAGCTTCTCTTCCGAAGTCATCTG-3' [SEQ ID NO: 2]
[0071] The amplified sequence of 538bp is shown in Table 1 (SEQ ID NO: 21)
[0072] The amplified DNA contains the essential origin (ori) and packaging sequence (pkg). This kPCR fragment (KpnI-ori / pkg-HindIII) was digested with HindIII only and inserted into the pSKII vector, which was digested...
Embodiment 3
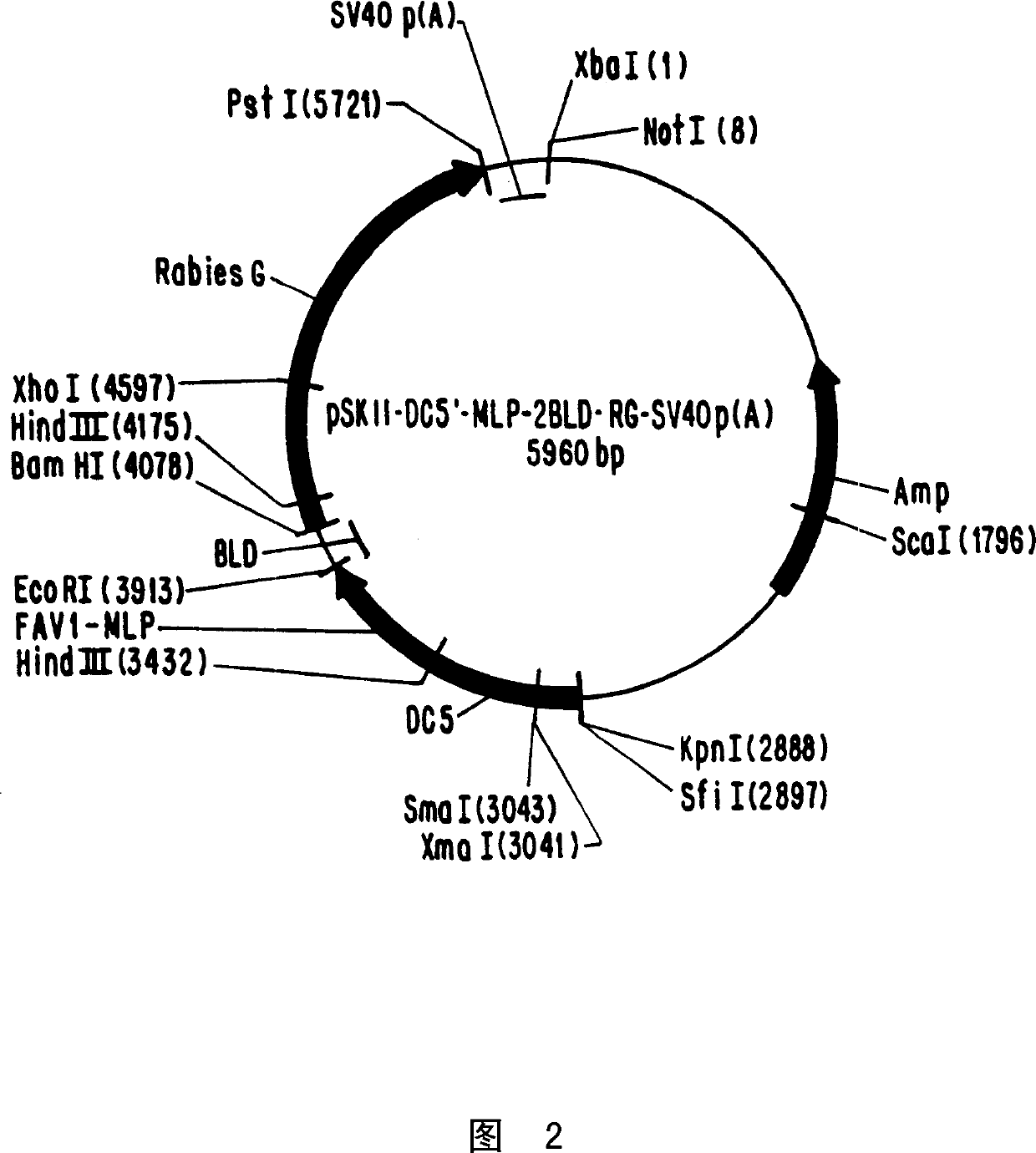
[0089] This example describes the generation of plasmid pSKII-DC5'-MLP-2BLD-RG-p(A), which is depicted in FIG. 2 . Plasmid pSKII-DC5'-MLP-2BLD-RG-p(A) was prepared in three consecutive steps by directional cloning. First, using oligonucleotide primers, reverse transcriptase (Amersham) and PCR, the glycoprotein gene of the virus was prepared from the RNA of the rabies virus vaccine strain Vnukovo-32, up to 1640bp of the rabies virus DNA sequence was amplified and separate. The primers used were:
[0090] 5'-GGATCCAGGAAAGATGGTTCCTCAGGCTCTCCTGTTTG-3' [SEQ ID NO: 9]
[0091]5'-GCTGCAGCAAGGGGAGGTGATCTTCAGACTTGGATCGT-3' [SEQ ID NO: 10]
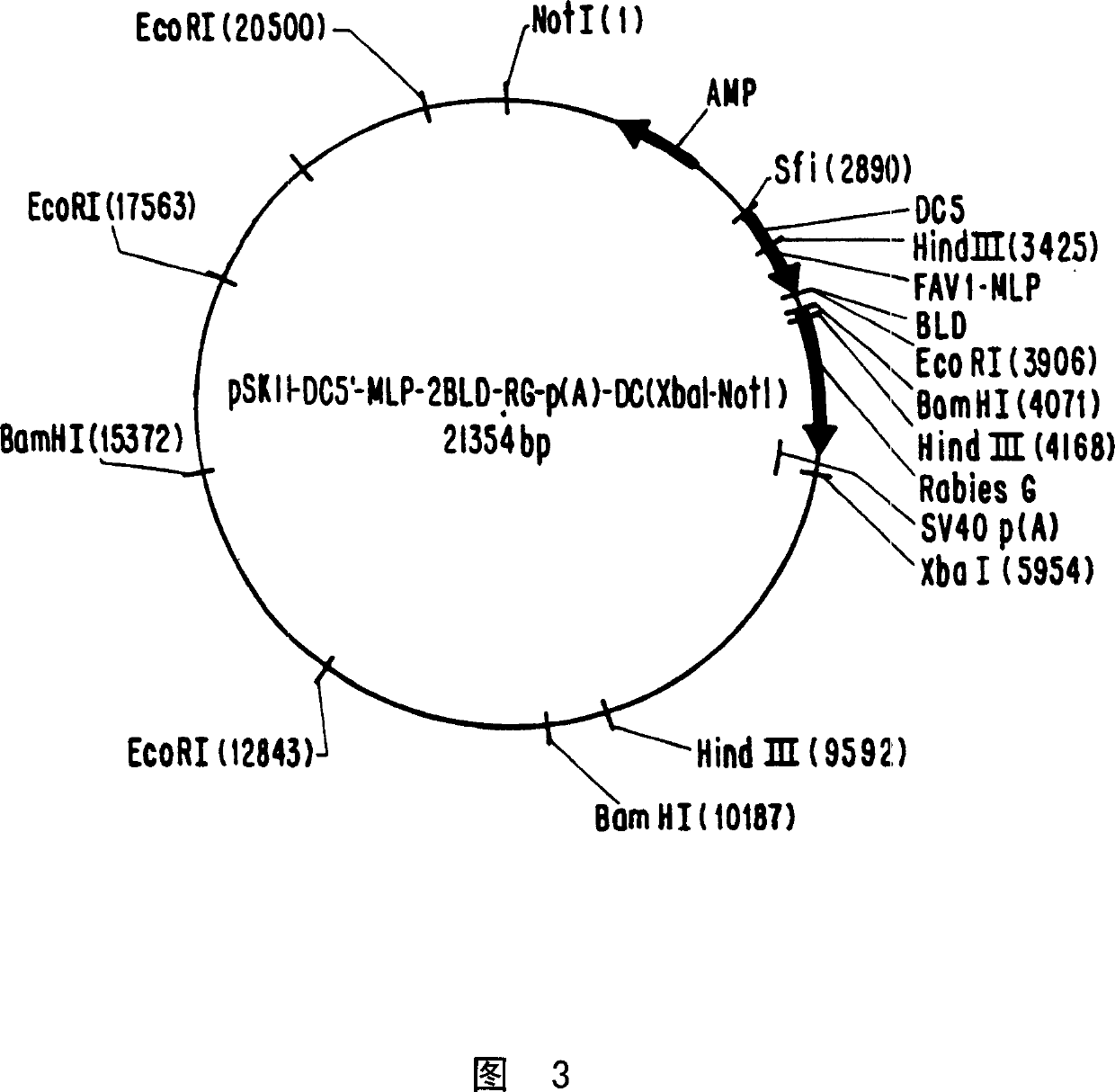
[0092] The amplified DNA contains the glycoprotein gene of the rabies virus vaccine strain Vnukovo-32 sequence (RG). The pSKII vector was subsequently opened with BamHI-PstI, and the PCR fragment BamHI-RG-PstI was inserted therein. In the second step, using oligonucleotide primers and polymerase chain reaction (PCR), up to 240 base pairs (bp)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com