Process for preparing uniform molecule weight polymerized hemoglobin
A technology of hemoglobin and molecular weight, which is applied in the field of biomedicine, can solve the problems of expensive cross-linking agent, difficult to buy, uneven molecular weight distribution of polymers, etc., and achieve the effect of overcoming the unevenness of polymerization products and the reduction of colloid osmotic pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of polymerized bovine hemoglobin with uniform molecular weight using Q Sepharose fast flow medium:
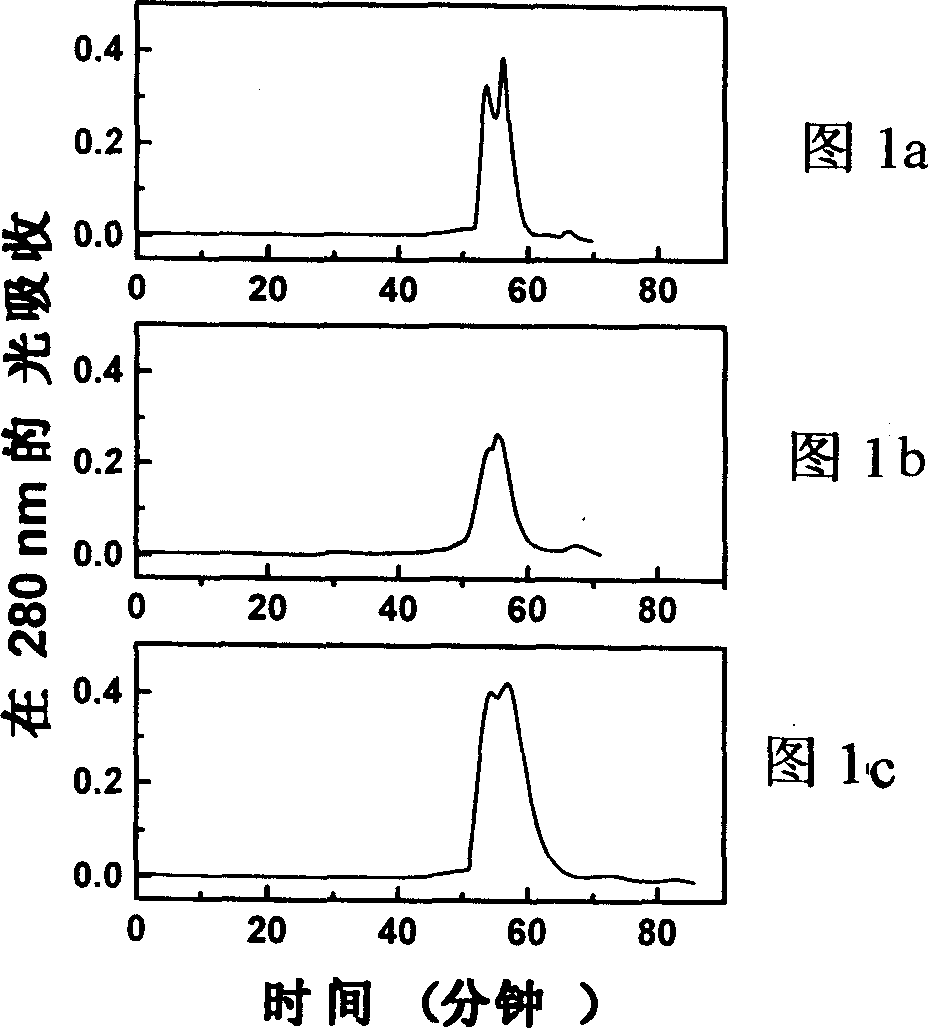
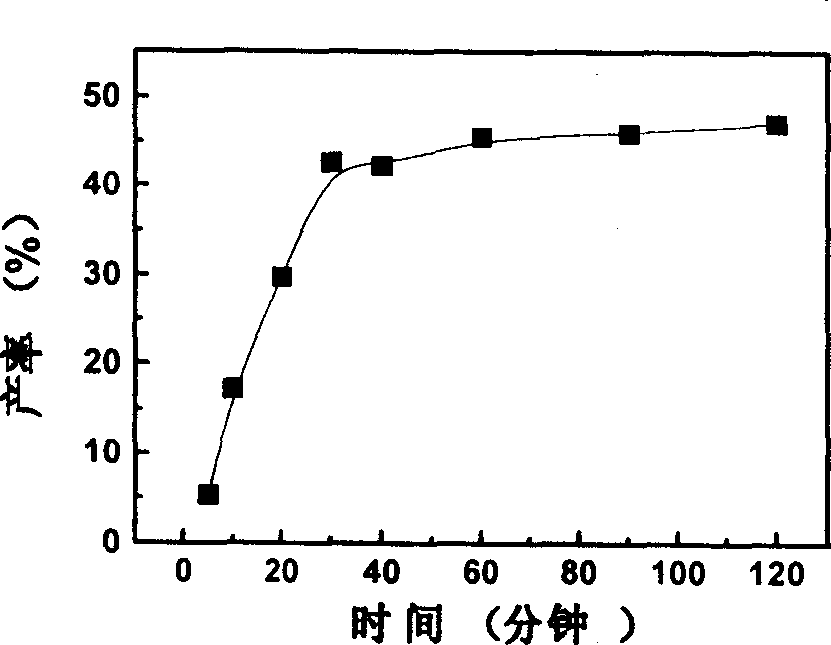
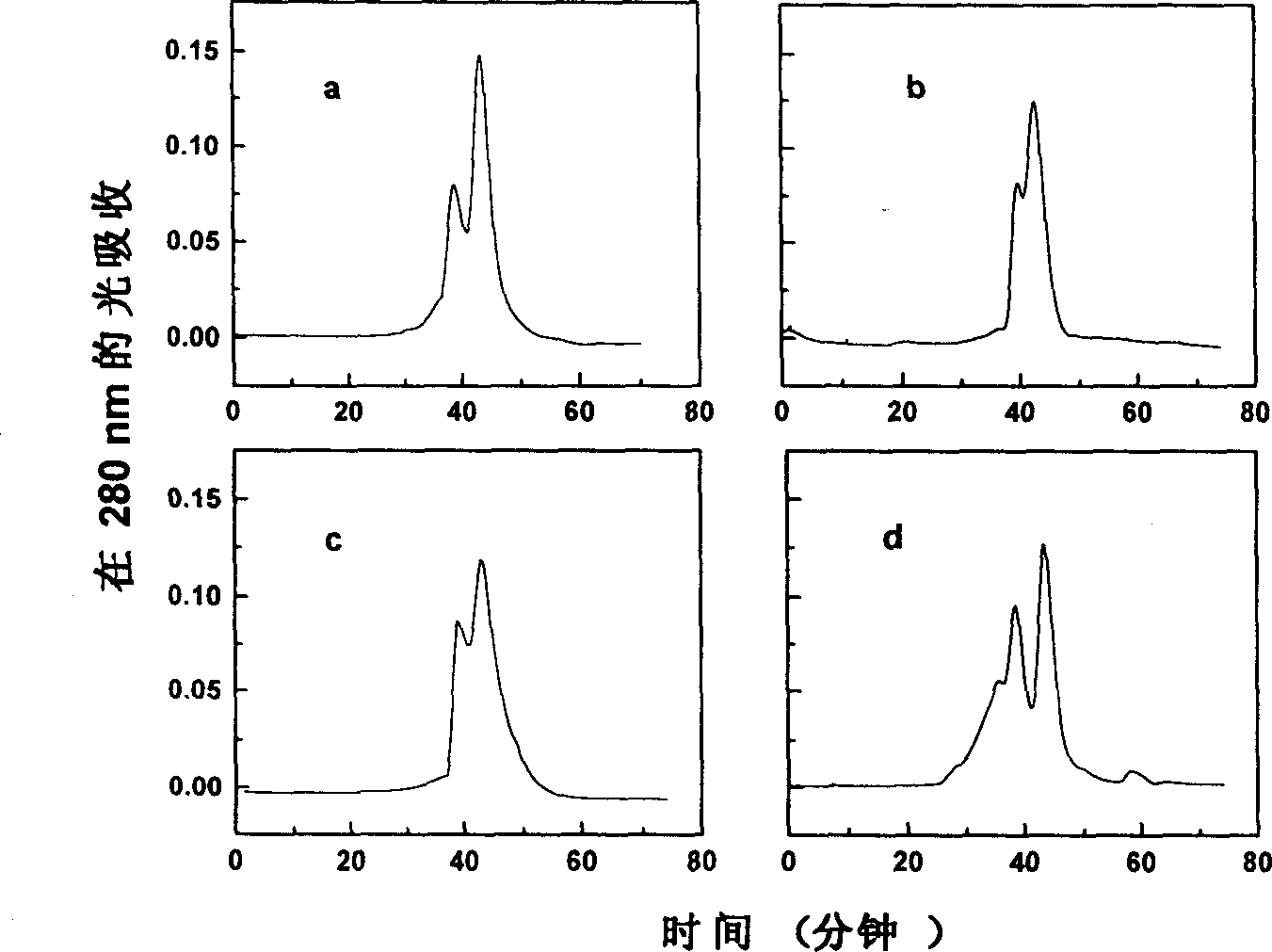
[0024] Fill the pure bovine hemoglobin solution with nitrogen at 4°C for 2 hours to make the bovine hemoglobin in a deoxygenated state; equilibrate the Q Sepharose fast flow medium with 20mM HEPES buffer (pH 8.0); pump to remove the oxygen in the Q Sepharose fast flow medium , then filled with nitrogen; take 15ml of treated solid phase and 10ml of deoxygenated hemoglobin (3mg / ml, pH8.0) and evenly mix; Glutaraldehyde solution was reacted at 4°C for 1 hour under nitrogen protection; then equilibrated with 10 column volumes of 20mM HEPES buffer (pH8.0); finally, with 20mM HEPES buffer (pH8.0) containing 0.4M sodium chloride .0) elution, the eluate was detected at 280nm, and the elution peak was collected carefully; after the collection was reduced with sodium borohydride, the salt was removed through a dextran G-25 column; the collection was placed on...
Embodiment 2
[0025] Example 2 Preparation of polymerized bovine hemoglobin with uniform molecular weight using Q agarose large particle medium:
[0026] The hemoglobin solution was filled with nitrogen at 4°C for 2 hours to make the hemoglobin in a deoxygenated state. The Q Sepharose macroparticle medium was equilibrated with 20 mM HEPES buffer (pH 8.0). Aspirate to remove oxygen from the Q Sepharose medium and flush with nitrogen. Take 15ml of the treated solid phase and mix evenly with 5ml of 6mg / ml deoxyhemoglobin (pH8.0). Subsequently, it was loaded into a 6.5×1 cm column, 0.5 ml of 0.4% (v / v) glutaraldehyde solution was added, and the mixture was reacted at 4° C. for 1 hour. Subsequently, equilibrate with 10 column volumes of 20 mM HEPES buffer (pH 8.0) at a flow rate of 1 ml / min. Finally, it was eluted with 20 mM HEPES buffer (pH 8.0) containing 0.4 M sodium chloride at a flow rate of 0.6 ml / min, and the eluted peaks were collected. After the collection was reduced with sodium bo...
Embodiment 3
[0027] Example 3 Preparation of polymerized hemoglobin with DEAE agarose fast flow medium:
[0028] Take 10 ml of 3 mg / ml hemoglobin solution (pH 8.0) and fill it with nitrogen gas at 4°C for 2 hours to make the hemoglobin in a deoxygenated state. The DEAE Sepharose fast flow medium was equilibrated with 20 mM HEPES buffer (pH 8.0). Aspirate to remove oxygen from the DEAE agarose fast flow medium. Take 15ml of processed DEAE agarose fast flow medium and mix with deoxygenated hemoglobin. The mixture was loaded into a 6.5×1 cm column, 0.5 ml of 0.4% (v / v) glutaraldehyde solution was added, and reacted at 4° C. for 1 hour under nitrogen protection. It was then equilibrated with 10 column volumes of 20 mM HEPES buffer (pH 8.0). Finally, it was eluted with 20 mM HEPES buffer (pH 8.0) containing 0.4 M sodium chloride, paying attention to collecting the elution peak. After the collection was reduced with sodium borohydride, it was passed through a Sephadex G-25 column to remove t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com