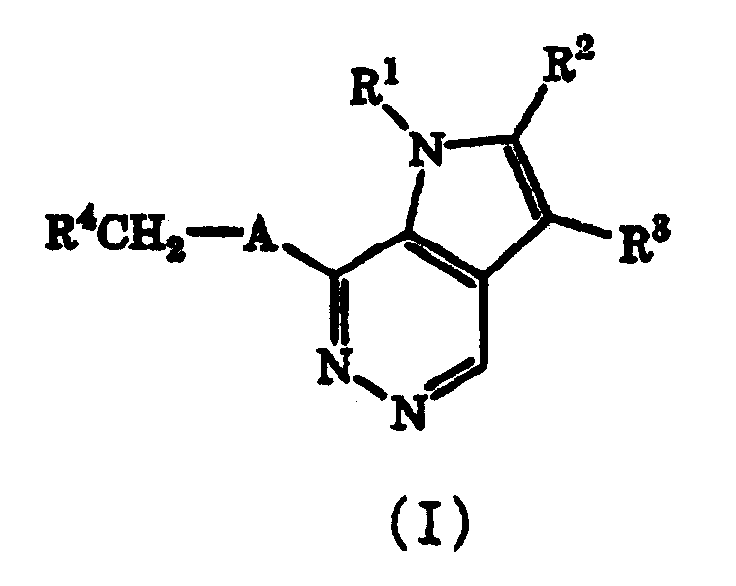
Pyrrolopyridazine compound
A technology of pyridazine derivatives and pyrrole, applied in the field of pyrrolopyridazine derivatives, can solve problems such as ulcer recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] 3-Acetoxymethyl-7-(4-fluorobenzyloxy)-2-methyl-1-[(1S,2S)-2-methylcyclopropylmethyl]pyrrolo[2,3-d ]Pyridazine
[0152] To 7-(4-fluorobenzyloxy)-2,3-dimethyl-1-[(1S,2S)-2-methylcyclopropylmethyl]pyrrolo[2,3-d at room temperature ] A solution of 0.679 g (2.00 mmol) of pyridazine in acetic acid (40 ml) was added with 6.58 g (12.0 mmol) of ammonium cerium(IV) nitrate, and then stirred at 60°C for 3 hours. The reaction mixture was poured into water, extracted with ethyl acetate, the extract was washed with saturated brine, and then dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography (solvent: hexane / ethyl acetate = 1 / 1). The obtained oil was crystallized from hexane to obtain 0.225 g (28%) of the title compound as light yellow crystals. .
[0153] Melting point: 122-123°C
[0154] Mass spectrum (CI, m / z): 398 (M + +1).
[0155] NMR spectroscopy (CDCl 3 , Δppm): 0.13-0.20 (m...
Embodiment 2
[0157] 7-(4-Fluorobenzyloxy)-3-hydroxymethyl-2-methyl-1-[(1S,2S)-2-methylcyclopropylmethyl]pyrrolo[2,3-d] Pyridazine
[0158] To 7-(4-fluorobenzyloxy)-2,3-dimethyl-1-[(1S,2S)-2-methylcyclopropylmethyl]pyrrolo[2,3-d at room temperature ] A solution of 67.9 g (200 mmol) of pyridazine in acetic acid (800 ml) was added with 329 g (600 mmol) of ammonium cerium(IV) nitrate, followed by stirring at 55°C for 8 hours. Water was added to the reaction mixture, extracted with ethyl acetate, the extract was washed with saturated brine, and then dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, methanol (500 ml) and 2N lithium hydroxide aqueous solution (160 ml) were added to the residue, and the mixture was stirred at room temperature for 40 minutes. The reaction mixture was neutralized with 1N hydrochloric acid, methanol was distilled off under reduced pressure, and extracted with chloroform. The extract was washed with saturated brine, and then dried ...
Embodiment 3
[0164] 7-(4-Fluorobenzyloxy)-3-formyl-2-methyl-1-[(1S,2S)-2-methylcyclopropylmethyl]pyrrolo[2,3-d]pyridin Oxazine
[0165] To 7-(4-fluorobenzyloxy)-3-hydroxymethyl-2-methyl-1-[(1S,2S)-2-methylcyclopropylmethyl]pyrrolo[2, 3-d] A solution of 64.3 g (181 mmol) of pyridazine in dichloromethane (900 ml) was added with 472 g (5.43 mol) of activated manganese dioxide, and then stirred at room temperature for 18 hours. The reaction mixture was filtered through Celite (trade name), the filtrate was concentrated under reduced pressure, and the resulting crude crystals (45.7 g) were washed with ethyl acetate and hexane to obtain 44.3 g (69%) of the title compound as pale yellow crystals.
[0166] Melting point: 138.5-139.5°C
[0167] Mass spectrum (CI, m / z): 354 (M + +1).
[0168] NMR spectroscopy (CDCl 3 , Δppm): 0.19-0.26 (m, 1H), 0.40-0.47 (m, 1H), 0.71-0.78 (m, 1H), 0.84-0.91 (m, 1H), 0.92 (d; J=5.9Hz, 3Hz ), 2.75(s, 3Hz), 4.19(dd; J=14.6Hz, 7.1Hz, 1H), 4.35(dd; J=14.6Hz, 6.6Hz, 1H), 5.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com