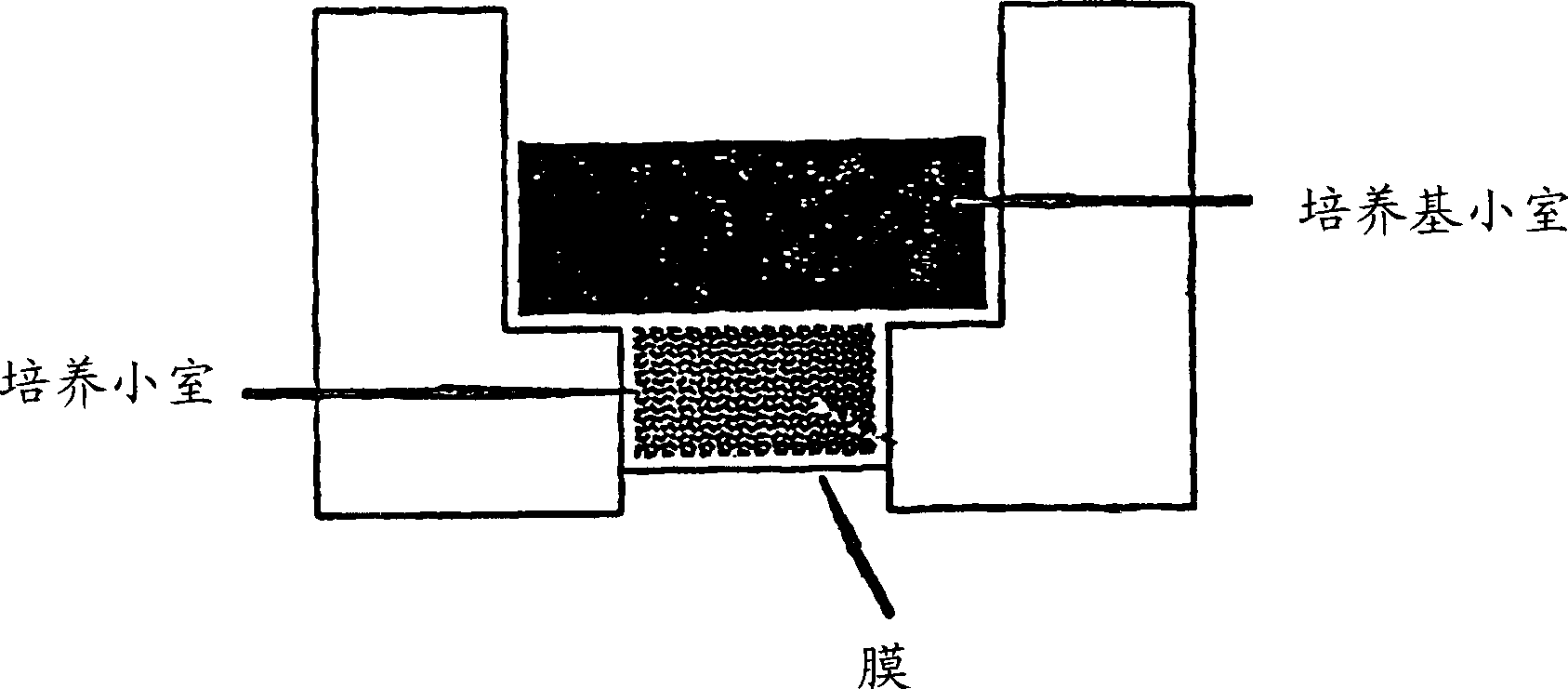
Human ex vivo immune system
A technique of immune system, culture system, applied in the field of methods and components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] General Materials and Methods
[0149] Flow Cytometry - Hemocytometer Analysis
[0150] Lymphocyte subtypes (helper and cytolytic T cells and B cells) and percentages of activated lymphocyte surface markers were quantified by flow-cytometry on an EPICS Profile Analyzer (Coulter, Miami, FL). Cell samples are incubated with fluorescently labeled antibodies and isotype controls. Antibodies used were anti-CD3 (pan T cells), anti-CD4 (helper T cells), anti-CD8 (cytolytic T cells), anti-TCRαβ (with αβ T cell receptor T-cells), anti-TCRγδ (T-cells with γδ T cell receptor), anti-CD45RA (naive T cells), anti-CD45RO (activated T cells), anti-CD19, anti - CD20, anti-CD21, and anti-CD10 (B cells) (10). Immunocytochemistry
[0151] Acetone-fixed cytospin slide preparations of nonadherent cells in culture were detected by monoclonal (anti-CD3, anti-CD19, anti-CD56, and anti-TdT) or polyclonal antibodies (anti-cytoplasmic μ, anti-surface IgG, and anti-surface IgM) labeling, ...
Embodiment 2
[0165] 3D human long-term bone marrow culture
[0166] An appropriate number of monocytes (4-6×10 6 The porous microcarrier portion transferred into the bioreactor per culture chamber) is incubated with the culture. The cultures were grown in a humid incubator (containing 5% CO 2 ) at 37°C. LTBMC culture medium (changed every day), by 70% (v / v) McCoy's 5A culture medium (Gibco), 1 * 10 -6M hydrocortisone (Sigma, St.Louis, MO), 50 u / ml penicillin (Sigma), 50 mg / ml streptomycin (Sigma), 0.2 mM L-glutamine (Gibco), 0.045% sodium bicarbonate ( Sigma), 1×MEM sodium pyruvate (Gibco), 1×MEM vitamin solution (Gibco), 0.4×MEM amino acid solution (Gibco), 12.5% (v / v) heat-inactivated horse serum (Gibco), and 12.5% Composition of heat-inactivated FBS (Gibco). The culture medium is supplemented with recombinant human stem cell factor (rhSCF 50ng / ml) and lymphocyte-specific lymphokines, IL-2 (rhIL2, 1000U / ml) and IL-7 (rh IL-7, 2ng / ml) . The cultures were fed daily with non-supp...
Embodiment 3
[0169] Detection of B lymphocytes
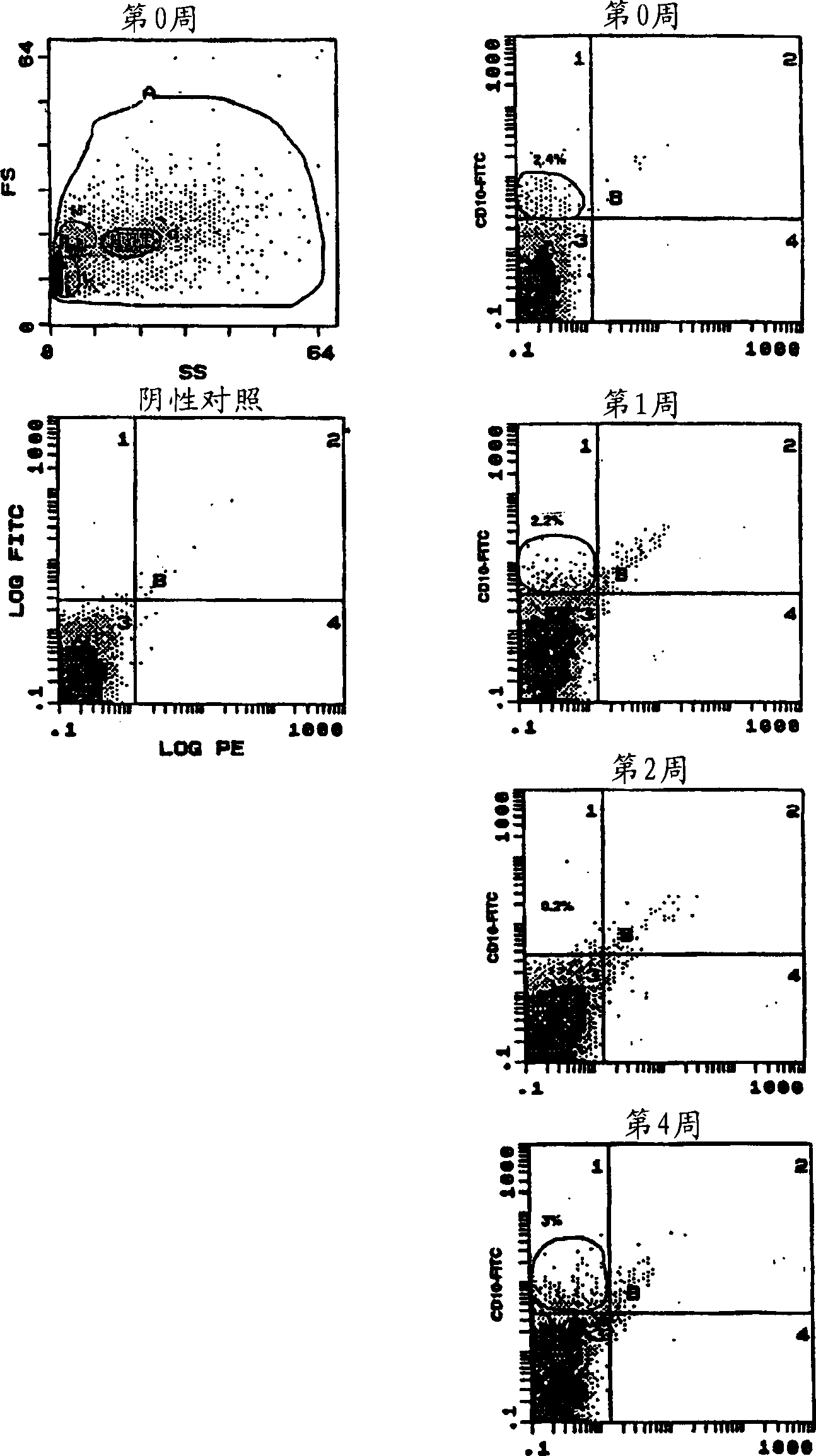
[0170] Flow cytometric analysis of cell outflow from three-dimensional human bone marrow simulations in the absence of exogenous growth factors identified pro-B (pro-B cells) (CD10 + ), immature B (CD19 + ), and mature B-cells (CD20 + , CD21 + )The presence. 2.4% of cells at week 0 expressed the marker CD10, which represented the pro-B (primary B cell) cell population in fresh bone marrow ( figure 2 ). The pro-B (pro-B cell) cell population was maintained at the same level after one week of culture. But at week 2, the pro-B (proto-B cell) cell population declined dramatically and only recovered at week 4. This fluctuation may represent the regenerative process that occurs in the three-dimensional culture and heralds the active B cell lymphopoiesis present in the bioreactor. Moreover, CD10 + B-cell populations (pro-B (pro-B-cell) cells) fluctuate with immature (CD19) and mature (CD20 + and CD21 + ) The fluctuations of B cells are...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com