APLIDINE treatment of cancers
A cancer, dose technology, applied in the direction of medical preparations containing active ingredients, depsipeptides, peptide/protein ingredients, etc., can solve the problems of ineffectiveness and limited treatment implementation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
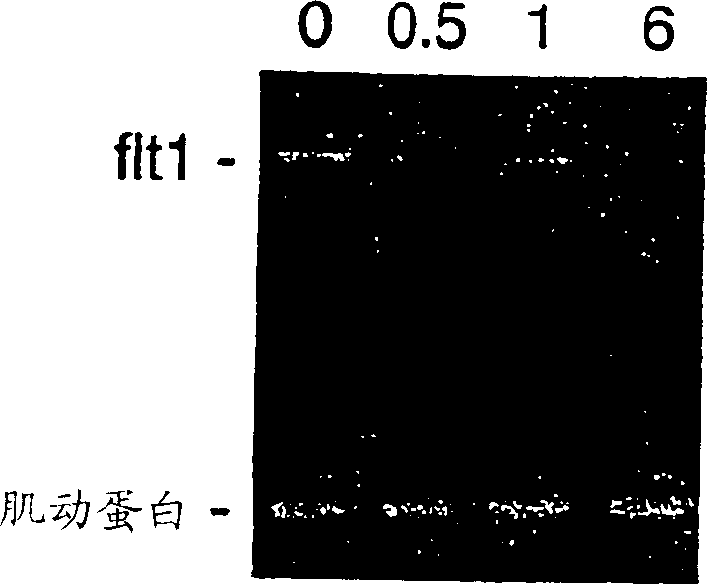
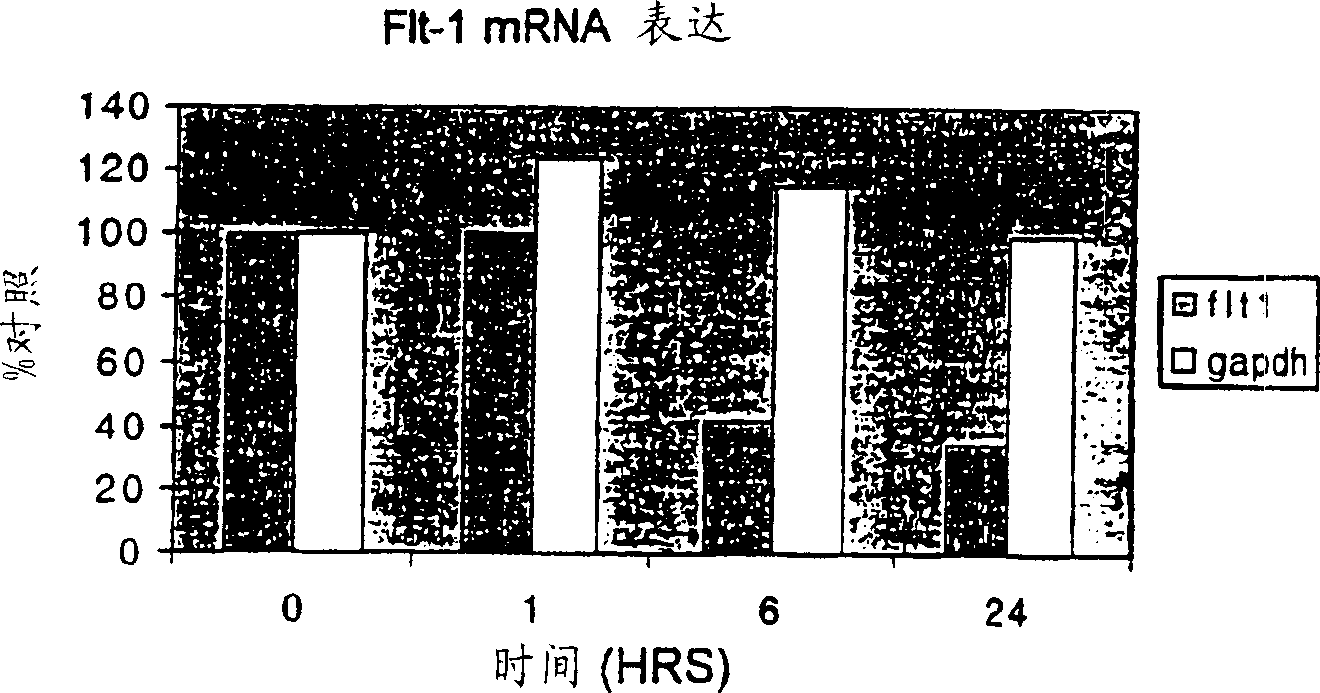
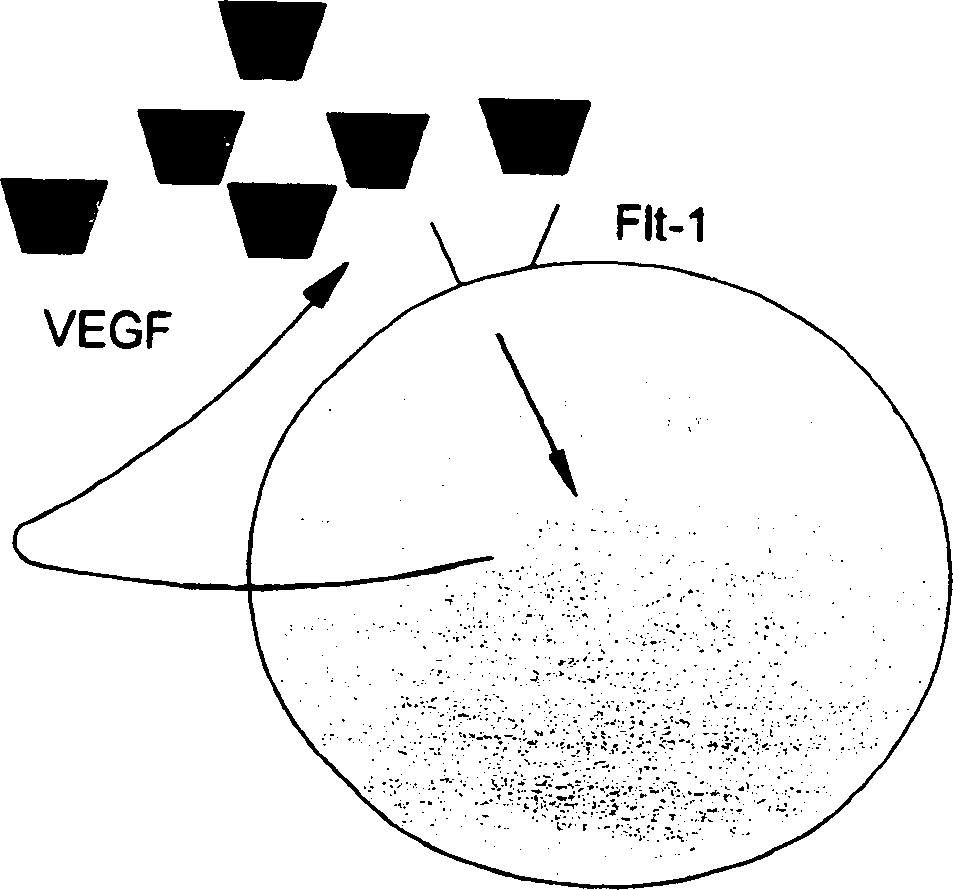
[0086] Gene expression patterns of human leukemia MOLT4 cells treated with the marine compound aplidine
[0087] Early changes in gene expression induced by aplidine in MOLT-4 cells were assessed by using cDNA expression arrays (Atlas Human Cancer, Clontech). MOLT-4 cells were treated for 1 hour with aplidine at a concentration that inhibited growth by 50%, and total RNA was isolated at 0, 1, 6 and 24 hours after drug elution. The filters were hybridized with equal amounts of 32P-labeled cDNA. The results were analyzed with ATLAS IMAGE 1.0 software. A change in gene expression of more than twofold was considered a significant change in RNA expression and was subsequently confirmed by PCR. A significant time-dependent decrease in VEGF-R1 (flt-1) expression was observed and confirmed at the RNA and protein levels by PCR and Western blotting, respectively.
Embodiment 2
[0089] Correlation of the selective antitumor activity of the marine-derived compound aplidine obtained with different model systems
[0090] Different model systems are evaluated to provide a basis for further clinical work. Selective antitumor activity was observed against two histologically distinct solid tumors: human gastric and prostate carcinomas. Potent in vitro activity against primary gastric tumor specimens or Hs746T gastric tumor cells was evident with ICs of 146 and 450 pM, respectively 50 value performance. It is judged that the IC50 activity on PC-3 prostate tumor cells is 3.4nM, the potency is low, but the selectivity is not low. In vitro activity in nude rodents was assessed using sc implanted tumor fragments or hollow fibers (HF) containing tumor cells.
[0091] the tumor
[0092] Optimal activity was observed after intraperitoneal administration in xenografted gastric (17-20%) and prostate (25-38%) tumors. Follow-up studies require intravenous ...
Embodiment 3
[0094] A phase I and pharmacokinetic study of weekly 24-hour infusions of aplidine in patients with advanced solid tumors
[0095] In vivo studies found increased in vivo activity by prolonging the duration of infusion. Sixteen patients were treated in this study. Patient characteristics were: mean age 55 years, mean PS 1, 11 / 5 male / female, tumor types: 5 head and neck tumors, 2 renal tumors, 3 colon tumors, 2 rectal tumors, sarcoma 1 case, 3 cases of melanoma, all patients had undergone chemotherapy pretreatment (average 2 lines).
[0096] Aplidine was administered as a 24-hour infusion at the following dose levels (Dls): 133 (3pts), 266 (3pts), 532 (3pts), 1000 (3pts), 2000 (3pts) and 3000 (1pts) mcg / m 2 / wk×3.
[0097] No dose-limiting toxicities (DLTs) were observed. Only non-hematological toxicities consisting of Grade 1 nausea, Grade 1 mucositis, and Grade 1 fatigue were reported. Phlebitis in the infusion arm was common and concentration-dependent. Pharmacokinet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com