Composition for treating hyperlipemia
A technology for hyperlipidemia and a composition, which is applied in the field of treatment of hyperlipidemia, can solve the problems of delayed disease treatment, single action mechanism, limited antihypertensive effect, etc., and achieves convenient use, long duration of curative effect and good comprehensive effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
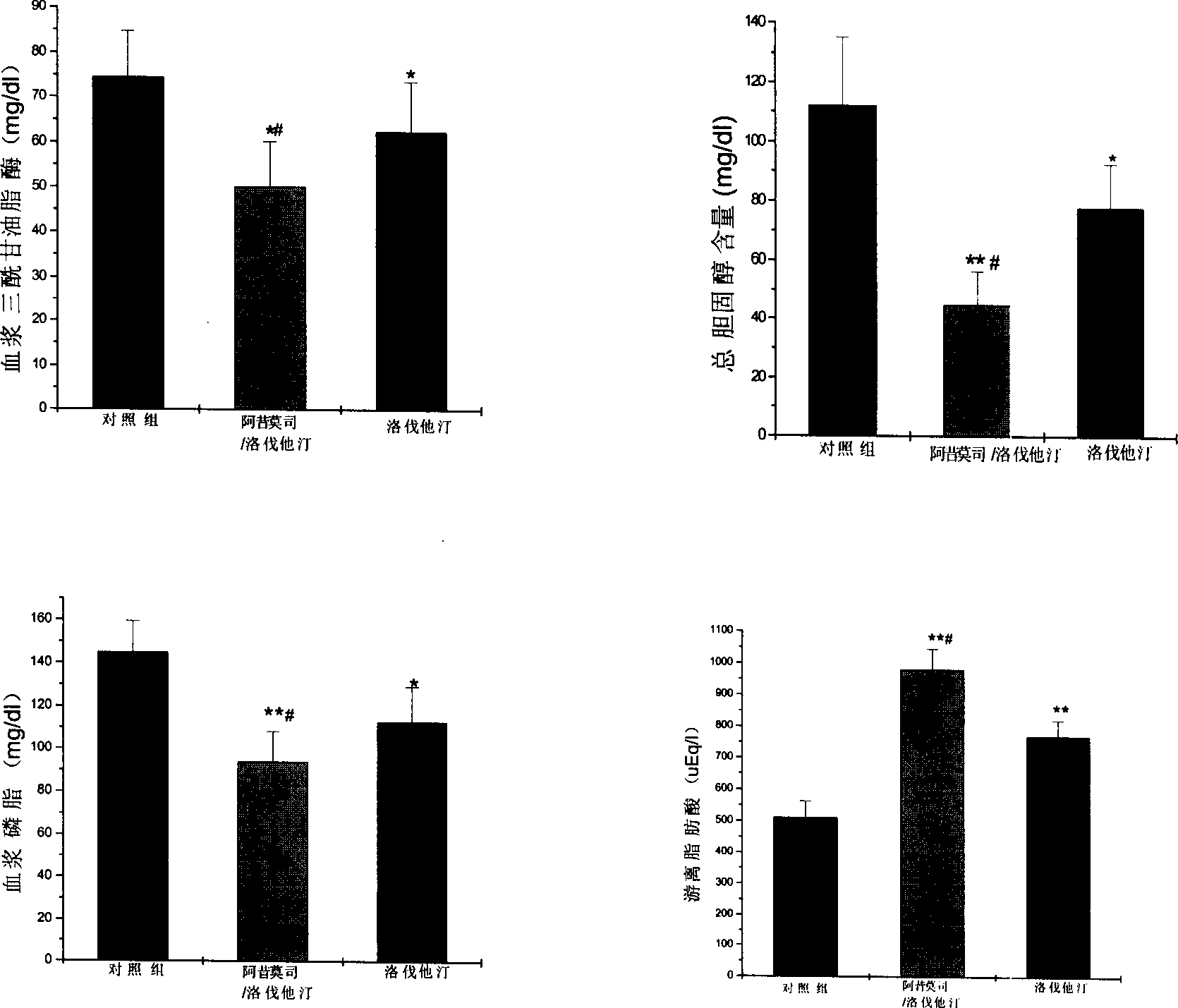
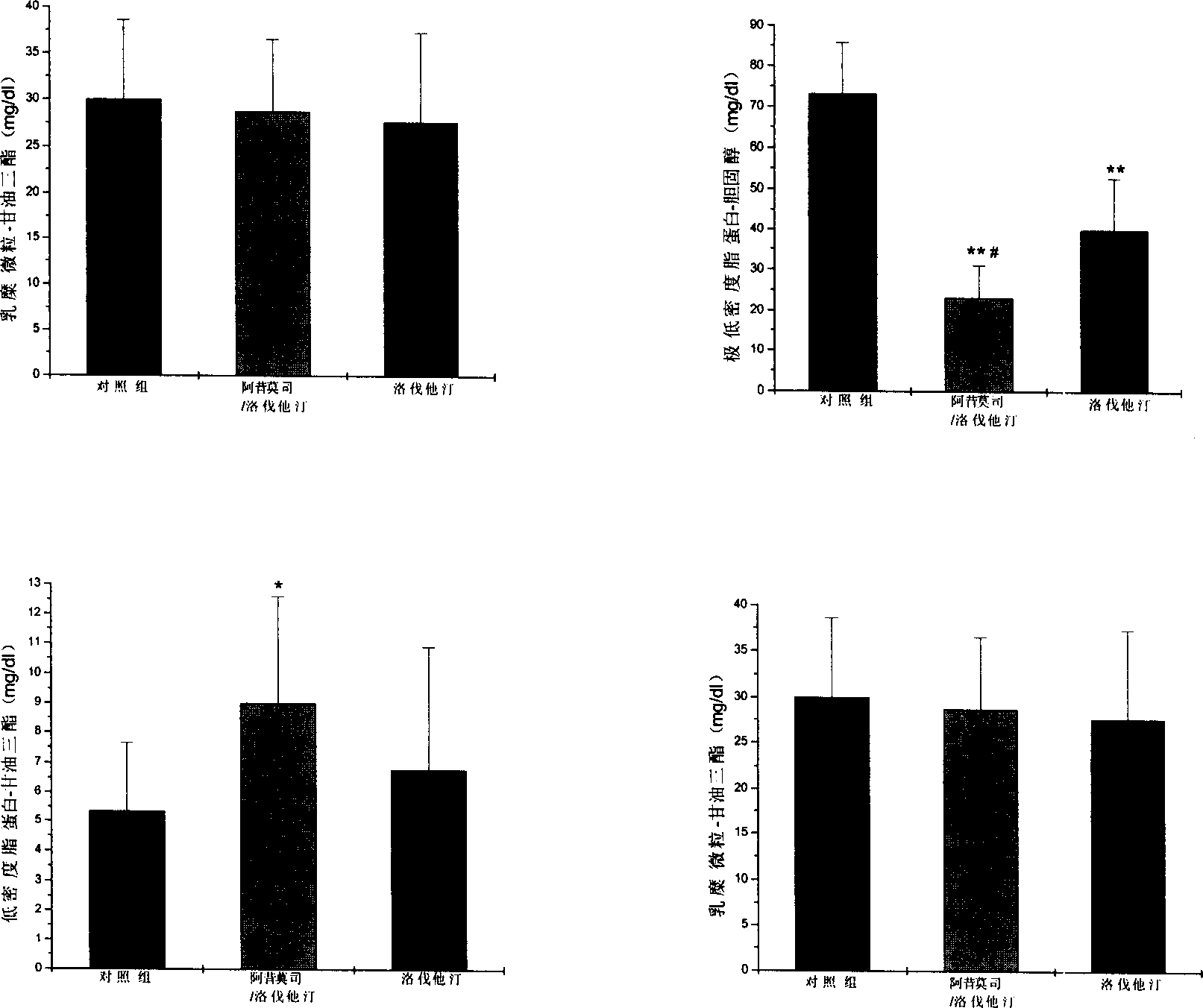
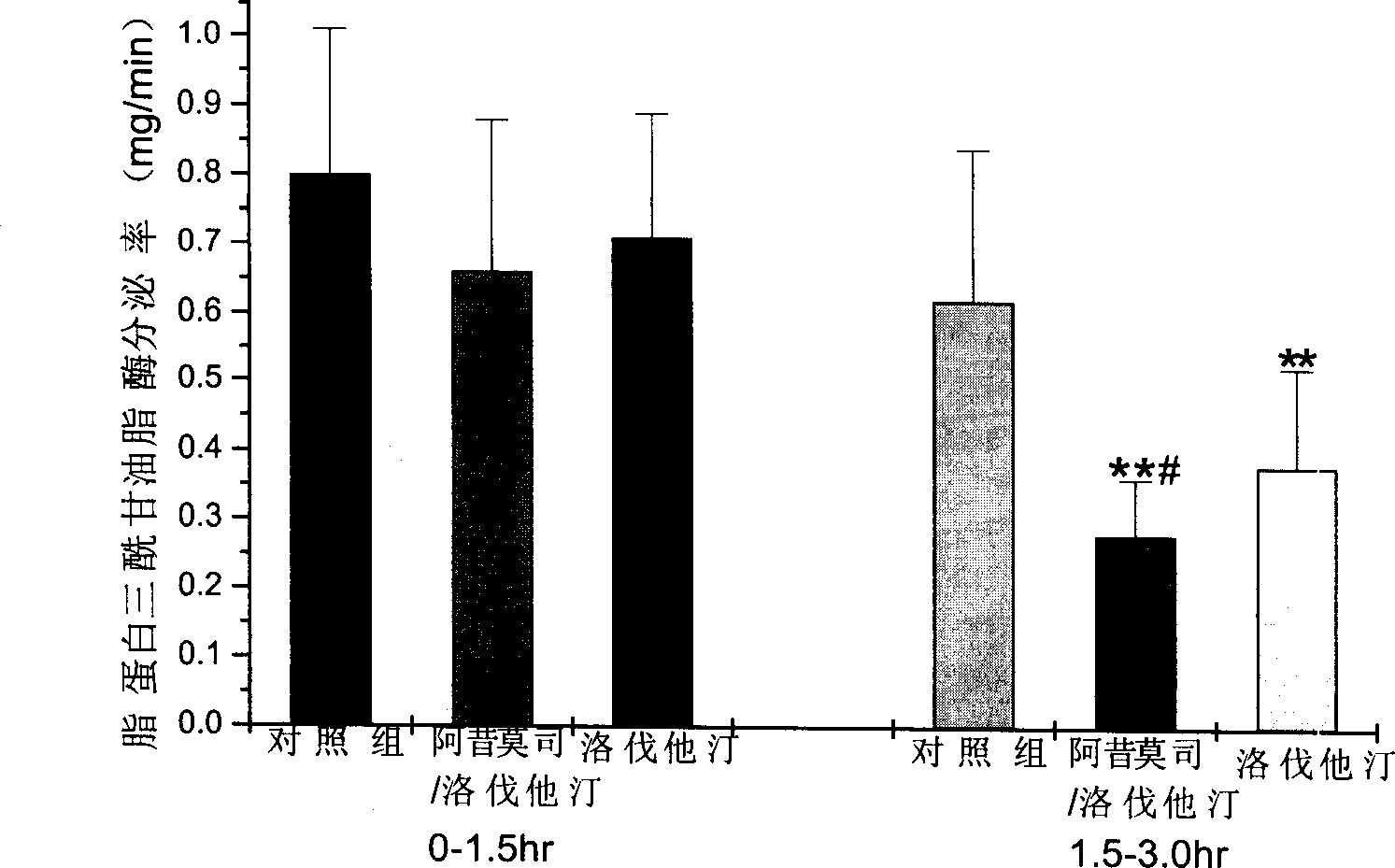
Image
Examples
example 1
[0031] Acipimus 250g
[0032] Lactose 30g
[0033] Sodium carboxymethyl starch 30g
[0034] Microcrystalline Cellulose 18g
[0035] 6% PVP in absolute ethanol 100g
[0037] Preparation process: Acipimox is passed through a 100-mesh sieve, lactose, sodium carboxymethyl starch, and microcrystalline cellulose are passed through a 80-mesh sieve, and the prescribed amount of acipimox, lactose, sodium carboxymethyl starch, and microcrystalline cellulose is weighed Mix the ingredients evenly, add an appropriate amount of 6% PVP absolute ethanol solution to granulate, dry at 60°C, sieve the dry granules with a 16-mesh sieve, and add the prescribed amount of magnesium stearate to the dry granules. b.
[0038] Lovastatin 10g
[0039] Hydroxypropyl Cellulose 30g
[0040] 20g pregelatinized starch
[0041] 6% PVP in absolute ethanol solution 50g
[0042] Glyceryl B...
example 2
[0045] Acipimus 375g
[0046] Hydroxypropyl Methyl Cellulose-4M 40g
[0047] Microcrystalline Cellulose 30g
[0048] 8% PVP in absolute ethanol solution 150g
[0050] Preparation process: Acipimox passes through a 100-mesh sieve, hydroxypropyl methylcellulose-4M and microcrystalline cellulose pass through a 80-mesh sieve, and the prescribed amount of acipimox and hydroxypropylmethylcellulose-4M is weighed , the microcrystalline cellulose is mixed evenly, add the absolute ethanol solution of 8%PVP and granulate in right amount, 60 ℃ of drying, 16 mesh sieve dry granules, add the magnesium stearate of recipe quantity in the dry granules. b.
[0051] Lovastatin 10g
[0052] Sodium Carboxymethyl Cellulose 30g
[0053] Lactose 20g
[0054] 6% PVP in 95% ethanol solution 50g
[0056] Preparation process: Lovastatin passes through a 10...
example 3
[0058] Niacin 500g
[0059] Mannitol 10g
[0060] Lactose 40g
[0061] Microcrystalline Cellulose 20g
[0062] 6% PVP in 95% ethanol solution 120g
[0063] Magnesium Stearate 2g
[0064] Preparation process: niacin is passed through a 100-mesh sieve, mannitol, lactose, and microcrystalline cellulose are passed through a 80-mesh sieve, and the prescribed amount of niacin, mannitol, lactose, and microcrystalline cellulose is weighed and mixed evenly, and 95% of 6% PVP is added. An appropriate amount of % ethanol solution is granulated, dried at 60° C., and the dry granules are sieved with a 16-mesh sieve, and the prescribed amount of magnesium stearate is added to the dry granules. b.
[0065] Lovastatin 10g
[0066] 25g pregelatinized starch
[0067] Mannitol 25g
[0068] 6% PVP in 95% ethanol solution 50g
[0069] Micronized silica gel 2g
[0070] Preparation process: pass lovasta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com