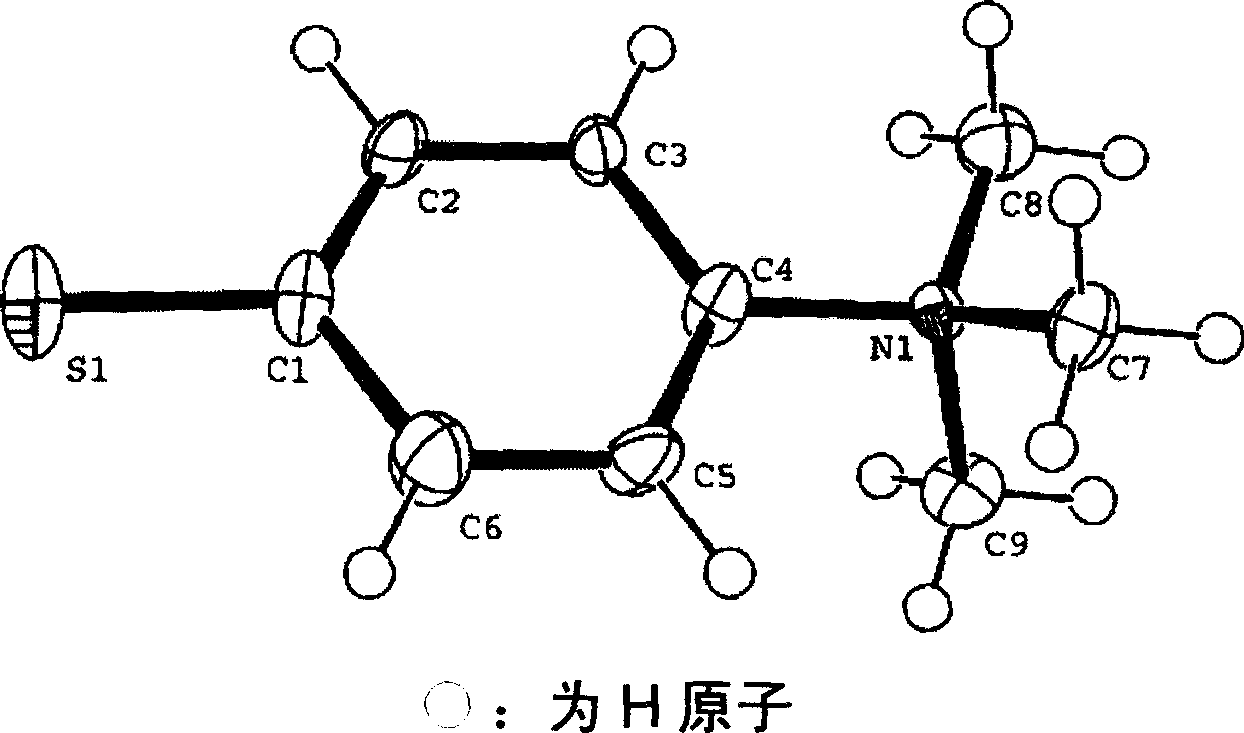
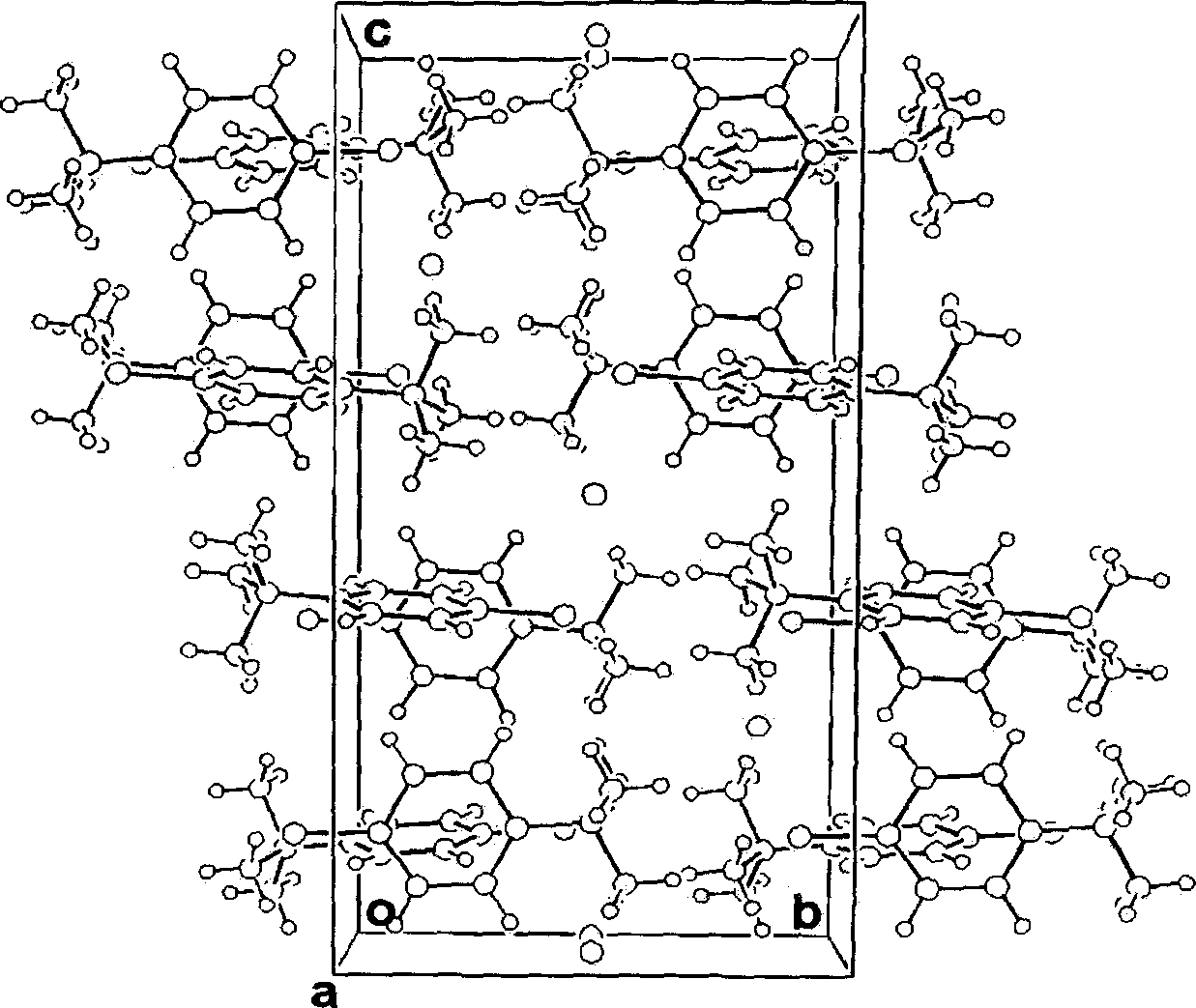
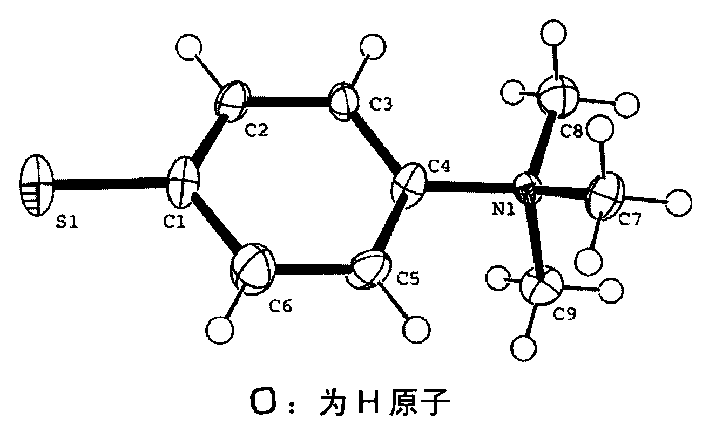
Amphiprotic compound containing sulphur, its preparing method and usage
A compound, amphoteric technology, applied in the field of amphoteric sulfur-containing compounds and their preparation, can solve the problems of undiscovered amphoteric sulfur-containing compounds, loss of organic reagents, environment, pollution, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Add 1.00 molar parts of dichlorodisulfide dropwise to 1.10 molar parts of N, N dimethylaniline in a benzene solution, stir for 2 hours to obtain a yellow sticky substance; then add 2.0 molar parts of methyl iodide acetone Solution; After stirring for 48 hours, a white solid was obtained by filtration, and the reaction condition was 0°C.
[0020] Dissolve the product obtained in the above steps in water, add 8.0 molar parts of hydrochloric acid and 5.0 molar parts of zinc powder, stir for 5.0 h, filter off excess zinc powder, add 1.8 molar parts of ammonium hexafluorophosphate to the solution, and stir for 1.0 hour, The white solid 4-trimethylammonium thiophenol hexafluorophosphate was obtained by filtration, and the reaction condition was 40±2°C.
[0021] Dissolve 1.0 molar part of 4-trimethylammonium thiophenol hexafluorophosphate in 150 ml of acetonitrile, add 1.0 molar part of triethylamine dropwise, filter and wash with ether to obtain a white powdery sol...
Embodiment 2
[0045] Example 2: Dissolve 1.0 molar parts of 4-trimethylammonium thiophenol hexafluorophosphate in 150 ml of acetonitrile, add 1.25 molar parts of triethylamine dropwise, filter and wash with ether to obtain a white powdery solid with a purity of 82.5%. . The reaction condition is 10±1°C, and the reaction time is 2.5h.
[0046] Dissolve the above-mentioned white powdery solid in a mixed solvent of 500ml of water and methanol (volume ratio 1:5) at room temperature, add diethyl ether dropwise, and precipitate colorless needle-like crystals, and dry them in vacuum at 40°C to obtain the product of the present invention. The rate is 77%.
Embodiment 3
[0047] Example 3: Dissolve 1.0 molar parts of 4-trimethylammonium thiophenol hexafluorophosphate in 150 ml of acetonitrile, add 1.5 molar parts of triethylamine dropwise, filter and wash with ether to obtain a white powdery solid with a purity of 89% . The reaction condition is 10±1°C, and the reaction time is 3h.
[0048] Dissolve the above-mentioned white powdery solid in a mixed solvent of 500ml of water and methanol (volume ratio 1:5) at room temperature, add diethyl ether dropwise, and precipitate colorless needle-like crystals, and dry them in vacuum at 40°C to obtain the product of the present invention. The rate is 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com