Novel organometallic compound having high metathesis activity and method for preparation thereof, metathesis reaction catalyst
An organometallic and metathesis polymerization technology, applied in the direction of ruthenium organic compounds, osmium organic compounds, platinum group organic compounds, etc., can solve the problems of catalyst dispersion affecting reaction rate, uneven polymer physical properties, and product safety problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~15
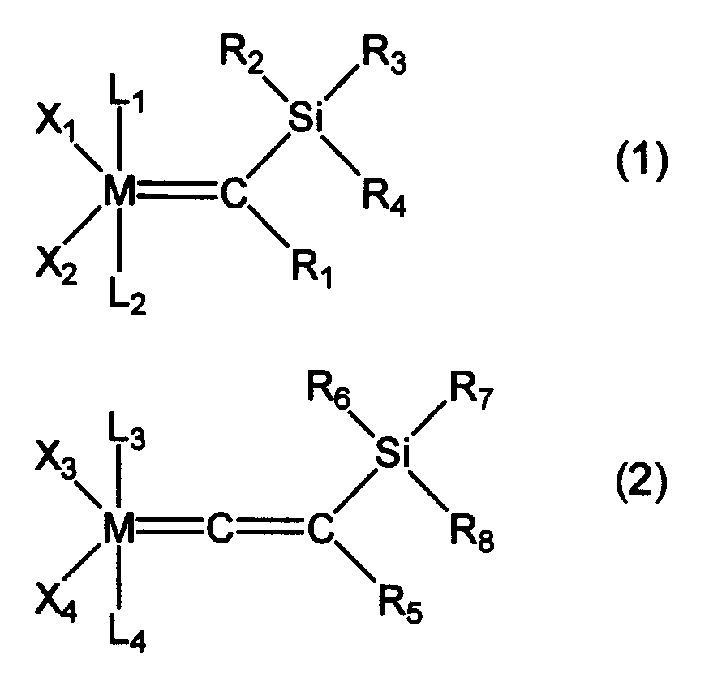
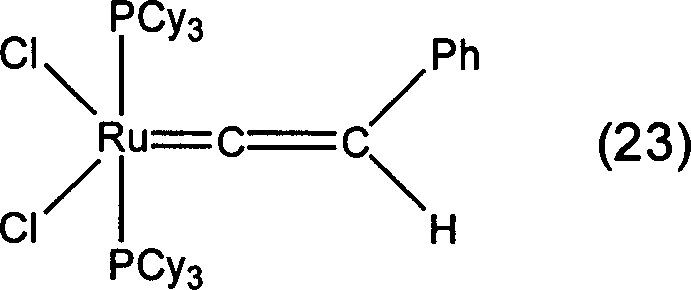
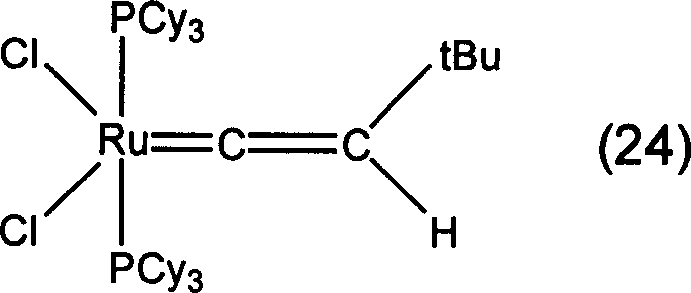
[0085] In Examples 1 to 15, organometallic compounds having structures represented by formulas (3) to (17) described later were synthesized according to any of the methods described in the above reaction formula (B) or (C). Table 1 shows the structural formulas of the organometallic compounds obtained in each example and the types of metal elements, ligands, substituents, etc. in the above-mentioned general formula (2).
[0086] In addition, in Example 1, as described in the following (A) to (B), the organometallic compound of formula (3) was synthesized according to the two methods of (B) and (C) above, but any method Both yield the same organometallic compound.
[0087] In addition, in Examples 2 to 13 and Example 15, except for changing the starting materials, the same steps as in Example 1 were used to experiment with any method of (B) or (C) to obtain each desired organometallic compound. In addition, in the method of (c), it has the disadvantage of being difficult to i...
Embodiment 16
[0116] In this example, an organometallic compound having a structure represented by the following formula (19) was synthesized according to the method described in the above-mentioned reaction formula (A).
[0117] 0.006 mol of RuCl 2 (PPh 3 ) 3 and 0.006 mol of trimethylsilyl diazomethane in 20 ml of dichloromethane, reacted at -78°C for 5 minutes. Next, the reaction solution was raised to room temperature, and 0.0132 mol of tricyclohexylphosphine was added to react for 30 minutes. After the reaction was completed, the solvent was removed under reduced pressure, and the complex was isolated by recrystallization in a dichloromethane / methanol system (yield 75%). As a result of analysis, it was judged to have the structure of formula (19) described later.
[0118] 1H-NMR (CDCl 3 )δ=0.20(s, 9H, -SiMe 3 ), 1.18, 1.49~1.72, 2.64 (multiple peaks, 66H, PCy 3 ), 19.8 (s, Ru=CH, 1H, Ph): 31P{1H} NMR (CDCl 3 )δ=19.60(s). Elemental analysis: C40H76Cl2P2SiRu: calculated value C...
Embodiment 17~19
[0122] In Examples 17 to 19, polymers obtained by performing metathesis polymerization of DCPD using the organometallic compound of the present invention were evaluated as follows.
[0123] Weigh 0.1m mol (DCPD1 / 10000 mol) of the organometallic compound of the present invention represented by the above formulas (3), (4), and (13), dissolve it in 0.5g of toluene, and add it to 132g of DCPD , set a 4mm-thick spacer to flow into the liquid, keep it at 80°C for 1 hour, then leave it at 120°C for 1 hour to obtain a polymer, and prepare a sample for physical property evaluation. The temperature of the tan δ peak of the obtained sample was measured by the dynamic viscoelasticity measurement method (measurement by the tensile method (n=1) of the viscoelastic spectrum data according to JIS K7198), and this was made into the glass transition temperature of the resin.
[0124] Next, a 0.5-g test piece was taken out from the obtained polymer, weighed in a 10-ml graduated flask, and toluen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com