Alkaline fuel battery with hydrogen storage alloy as electric catalyst
A technology of hydrogen storage alloy and electrocatalyst, applied in battery electrodes, circuits, electrical components, etc., can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
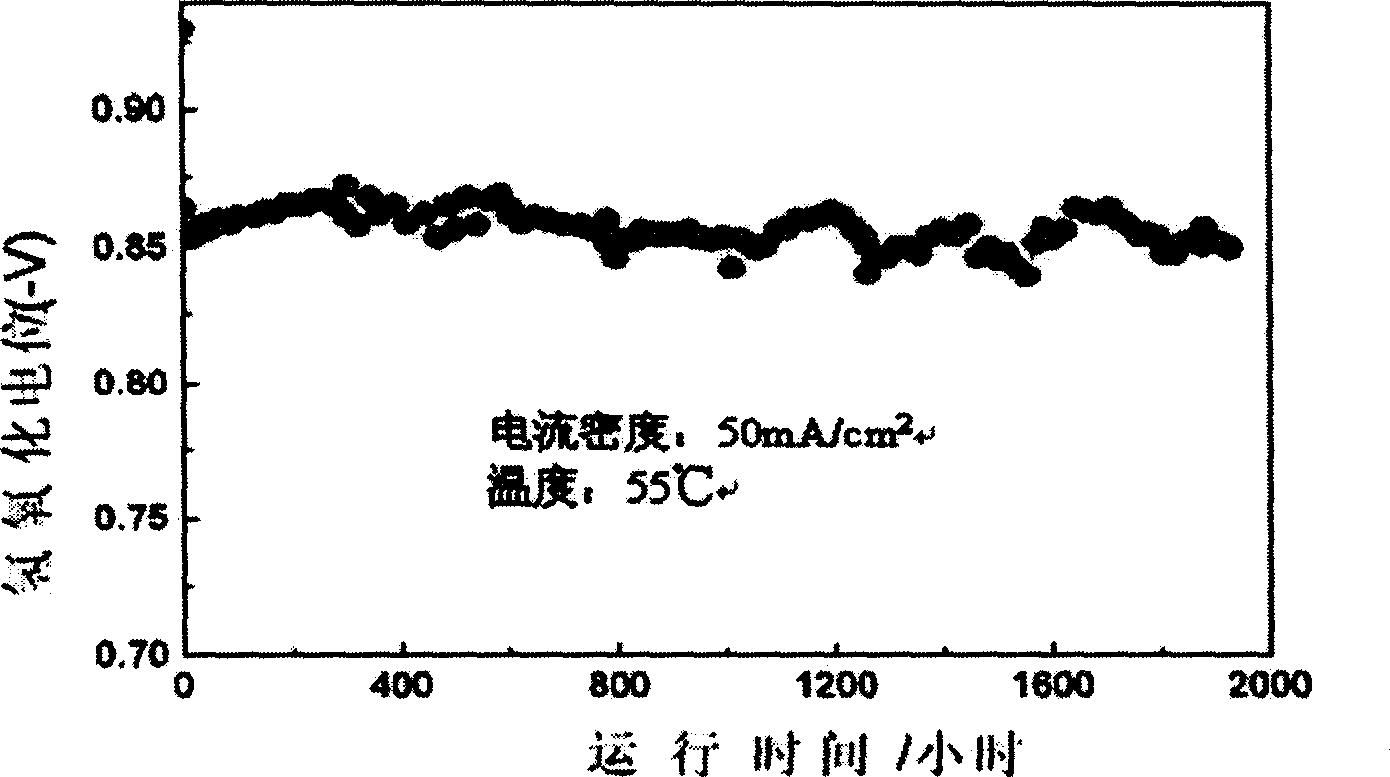
[0020] Preparation of AB by smelting 5 type hydrogen storage alloy ingot, its chemical composition is MmNi 3.35 co 0.75 mn 04 Al 0.3 (Mm is lanthanum-rich mixed rare earth). Then it is pulverized by mechanical method, and the alloy powder is prepared by hydrogenation method, and its particle size is 5-30 microns. With this hydrogen storage alloy powder and 10wt% PTFE coagulant, carbon black powder (its size is 0.25-0.40 micron; Specific surface area is 80m 2 / g) was mixed in a ratio of 80:10:10, and a hydrogen catalytic layer was formed on a metal nickel mesh by a rolling method, and its thickness was 0.4mm. Then in a hydrogen atmosphere, sinter at 280°C for 5 hours, and then with the gas diffusion layer at 200kg / cm 2 The hydrogen catalytic electrode is made under cold pressing. At 55°C, in 30wt% KOH solution, the current density is 50Ma / cm 2 , its hydrogen oxidation performance test results are shown in figure 1 .
Embodiment 2
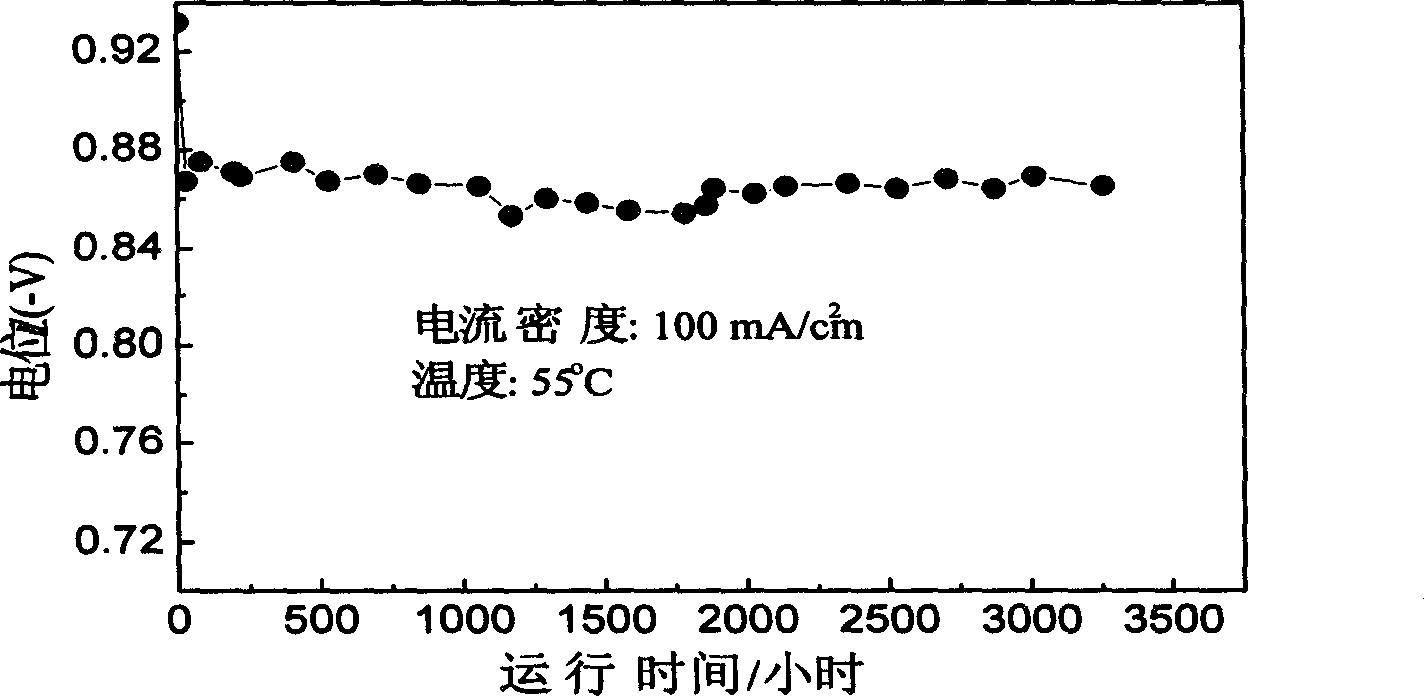
[0022] The preparation, chemical composition and pulverization process of the hydrogen storage alloy are the same as in Example 1. The particle size is then further reduced to 0.1-5 microns by mechanical ball milling. The preparation process of the hydrogen catalytic electrode is the same as in Example 1. At 55°C, in 30wt% KOH solution, the current density is 100mA / cm 2 , its hydrogen oxidation performance test results are shown in figure 2 .
Embodiment 3
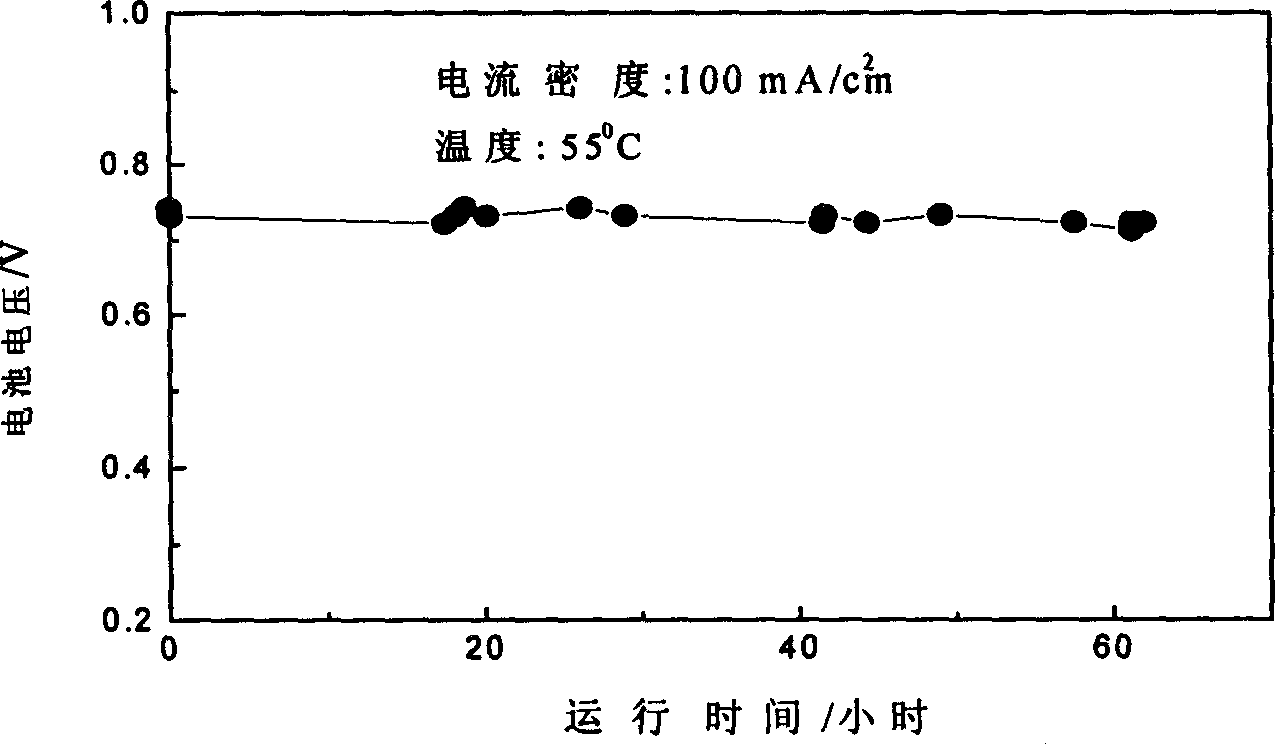
[0024] The hydrogen catalytic electrode is the same as in Example 2. The oxygen reduction electrode is made of La 0.6 Ca 0.4 CoO 3 Catalyst etc. The catalyst is prepared by co-precipitation method (citric acid is used as precipitating agent), and then calcined at 650° C. for 3 hours. The preparation process of the oxygen reduction electrode is similar to the hydrogen catalytic electrode, but the oxygen reduction electrode is in the inert gas N 2 Sintered in the atmosphere. The fabricated hydrogen catalytic electrode and oxygen reduction electrode constitute the anode and cathode of the fuel cell respectively. Use 30wt% potassium hydroxide as the electrolyte, asbestos membrane as the separator, the working temperature is 55°C, and the battery density is 100mA / cm 2 . see test results image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com