Synthesis of product prepared from imidazole acted with halogenated acetate ethyl ester
A technology of zoledronic acid and a new method, which is applied in the field of medicine, can solve the problems of high requirements for production personnel protection, three wastes treatment, and high equipment requirements, and achieve the effects of low cost, environmental protection, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
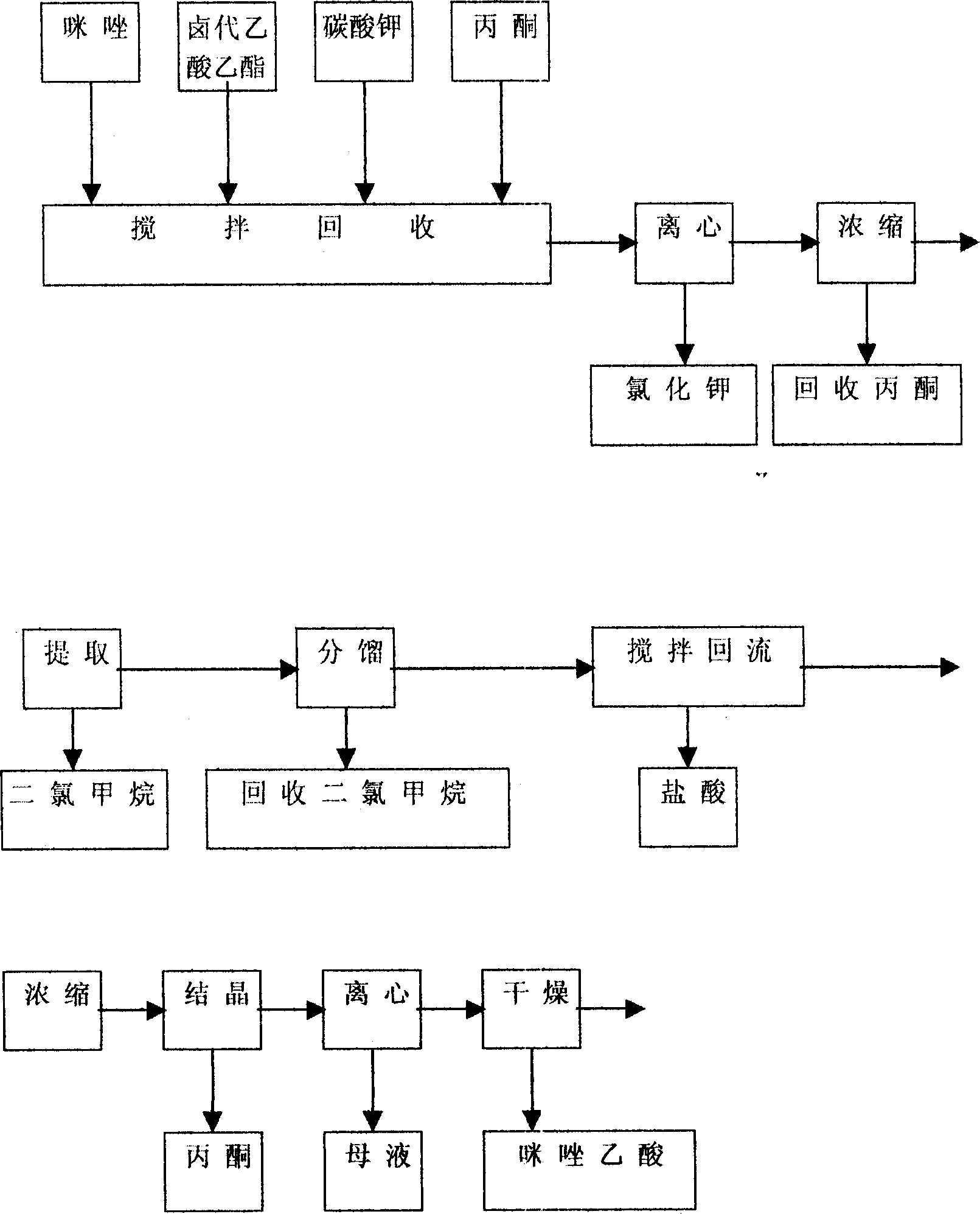
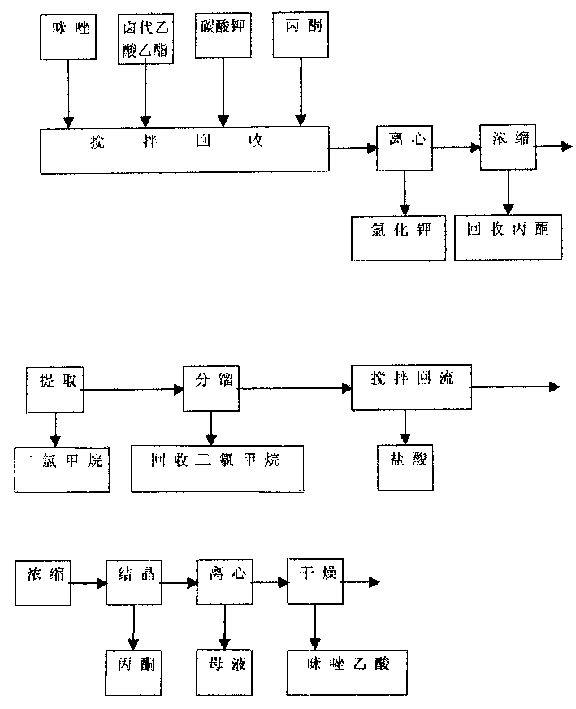
[0018] according to figure 1 Shown, the preparation of imidazole acetic acid:
[0019] Add 300 grams of imidazole and 450 milliliters of ethyl chloroacetate, 360 grams of anhydrous potassium carbonate, and 1500 milliliters of acetone into a 3000 milliliter three-necked reaction flask with stirring, condenser and thermometer, heat up to reflux, keep the reflux reaction for 6 hours, and cool to Filtrate at room temperature, recover acetone at normal pressure to dryness, add 600 ml of water to the residual oil, extract with dichloromethane, dry over anhydrous magnesium sulfate, recover the solvent at normal pressure to dryness, distill the residue under reduced pressure, collect 135-140 ° C / 5mmHg cut, obtains 220 grams of light yellow oily imidazole ethyl acetate.
[0020] Its reaction formula is:
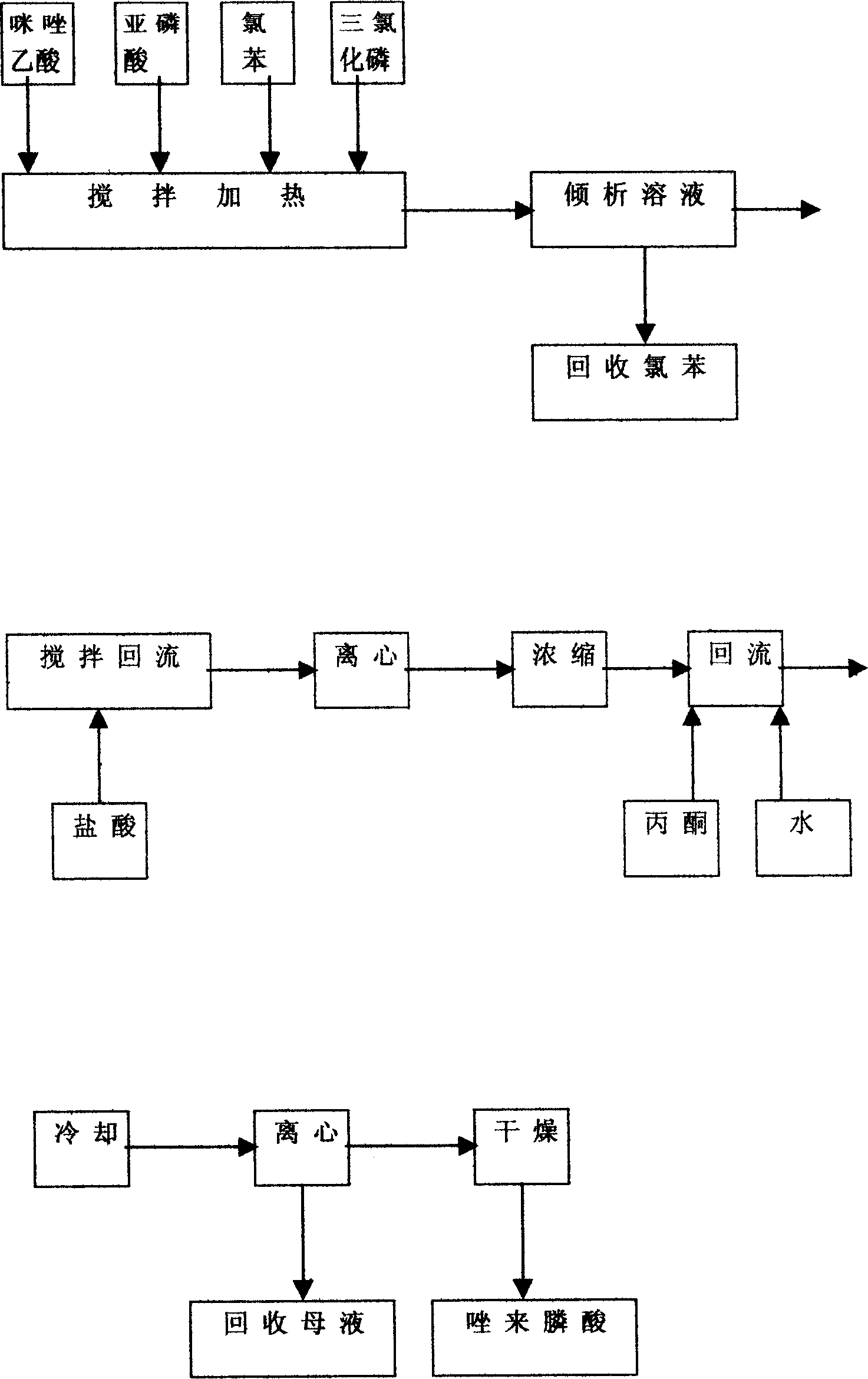
[0021] The obtained imidazole ethyl acetate is directly put into a 2000 ml three-neck reaction flask with a stirring and condenser tube without refining, and 900 ml of 4N hydroc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com