New earthworm kinase gene mutant
A technology of lumbrokinase and amino acid, which is applied in the field of new lumbrokinase cDNA mutants, can solve the problems of unclear reasons, unfavorable expression of lumbrokinase cDNA, and no report of lumbrokinase, and achieve the effect of high dissolution activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Stable expression of lumbrokinase mutant gene in Chinese hamster ovary cell line
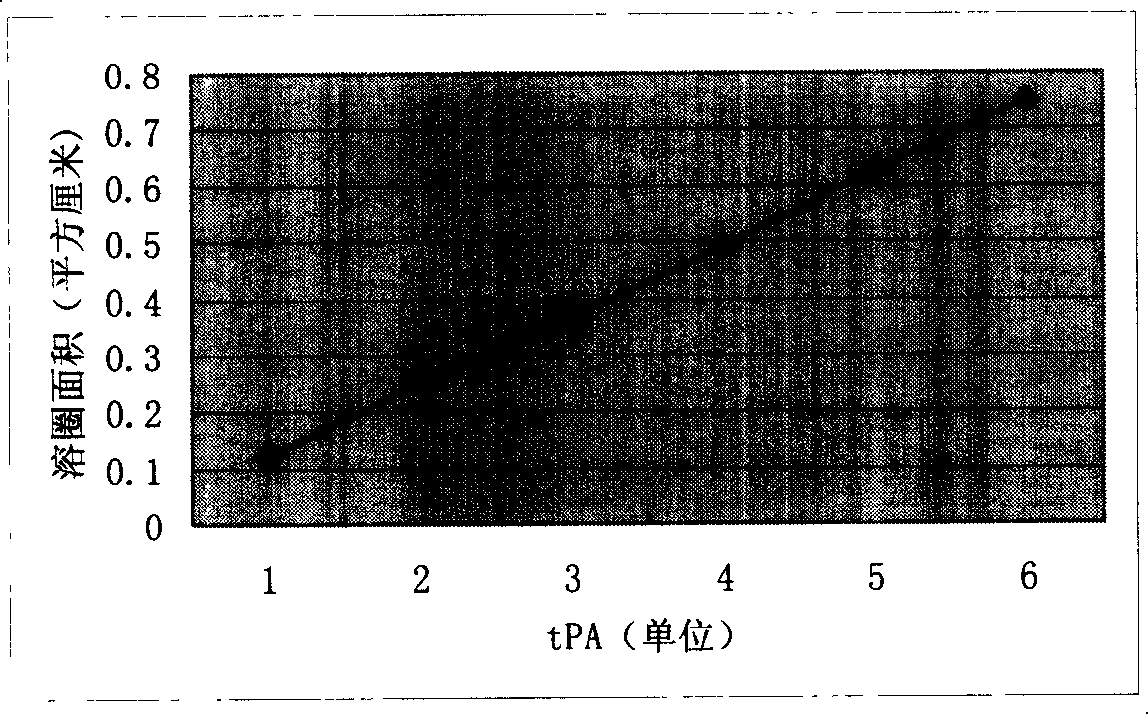
[0024] After the transformation of the lumbrokinase gene was completed by using the multi-site mutation PCR technique, in order to verify the expression ability of the mutant, this experiment constructed a eukaryotic expression vector of the lumbrokinase mutant gene, and transfected Chinese hamsters with liposomes Ovarian cell line (CHO), through G418 screening, obtained a CHO cell line stably expressing lumbrokinase, the expression product has obvious fibrinolytic activity, which can reach 500 tPA units per ml of cell culture supernatant.
[0025] 1. Construction of eukaryotic expression vector
[0026] The plasmid pMD18-LKm2 with the mutated target gene was digested with NotI and BamHI to recover a 0.86kb LKm2 fragment; then the pIRESlneo eukaryotic expression vector was digested with the same enzyme to recover a 5.23kb linearized vector. With a molecular ratio of 4:1, T4 DNA ligase wa...
Embodiment 2
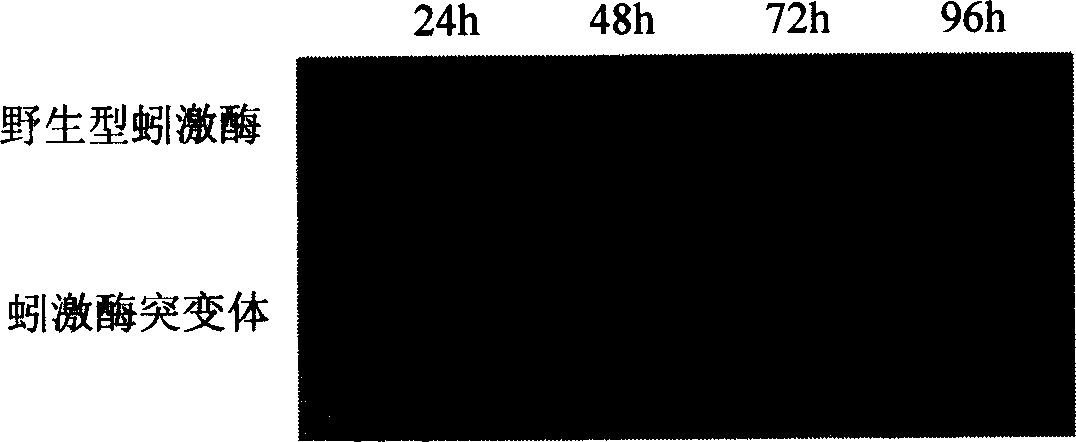
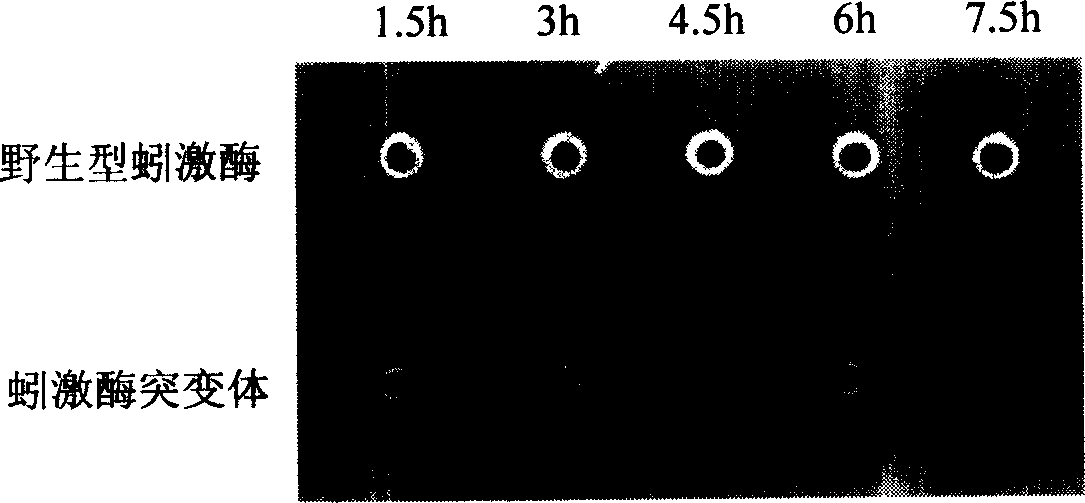
[0036] Comparison of temporal expression of lumbrokinase and its mutants in goat mammary gland
[0037] 1. Construction of eukaryotic expression vector for mammary gland localization
[0038] The plasmid pT-bCP was digested with SpeI+SacII, and the 1.13kb goat β-casein promoter sequence was recovered. The Klenow large fragment was filled in, and connected into the pIRESlneo vector plasmid that was digested with NruI+EcoRV to obtain the intermediate plasmid pI- bCP. Then pMD18-LK and pMD18-LKm2 were digested with NotI+BamHI, and the 0.86kb gene sequence of lumbrokinase and its mutants were respectively recovered, and connected into pI-bCP after the same digestion to obtain the mammary gland localization of lumbrokinase and its mutants Eukaryotic expression vectors pI-bCP-LK and pI-bCP-LKm2.
[0039] 2. Comparison of temporal expression of lumbrokinase and its mutants in goat mammary gland
[0040] After a large number of plasmids pI-bCP-LK and pI-bCP-LKm2 were prepared and p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com