Method for reutilization of residue from crude terephthalic acid product
A technology for terephthalic acid and crude products is applied in the field of recycling terephthalic acid crude product residues, and can solve the problems of limited recycling and utilization of CTA residues, high operating costs, complicated extraction processes and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
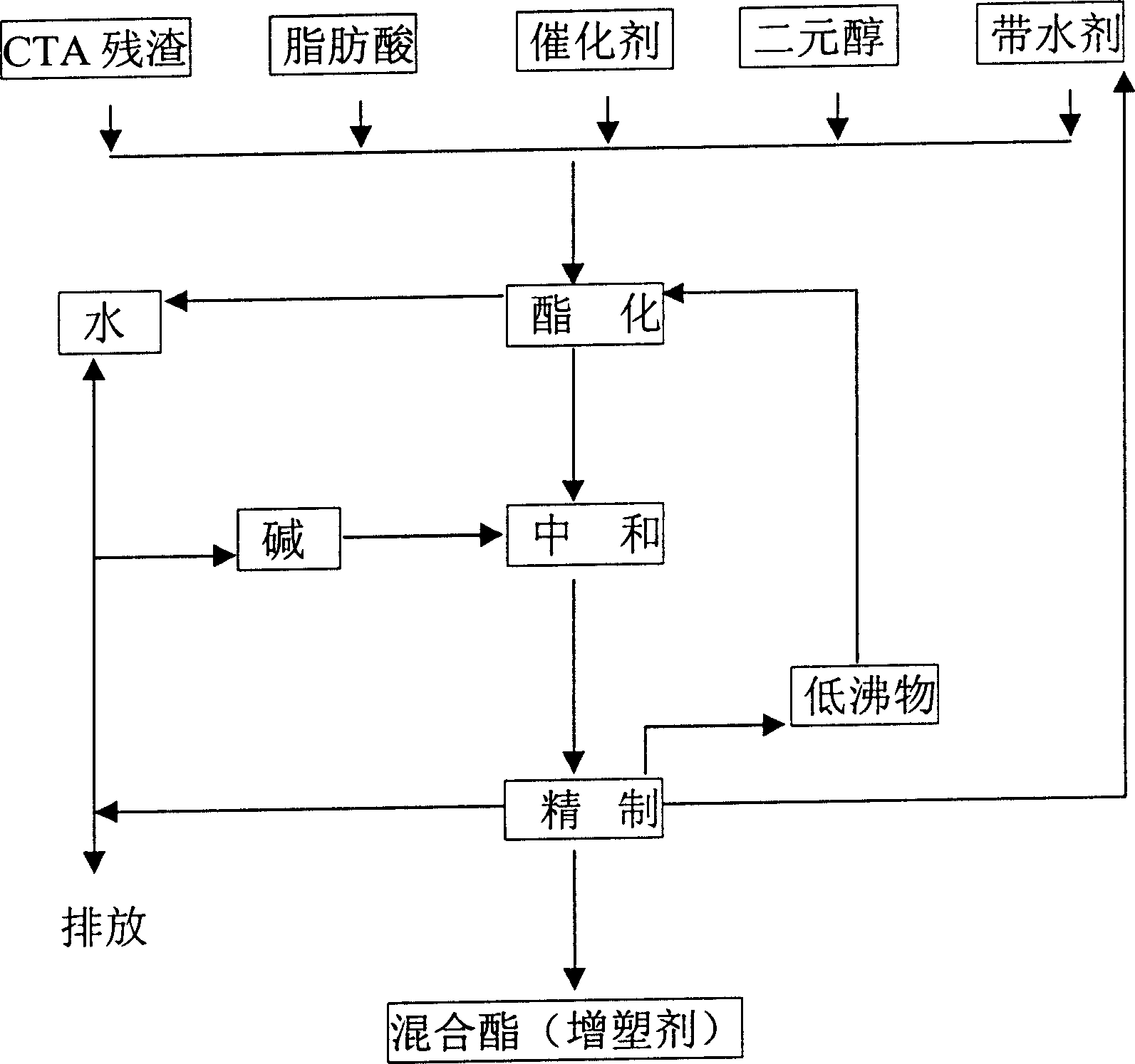
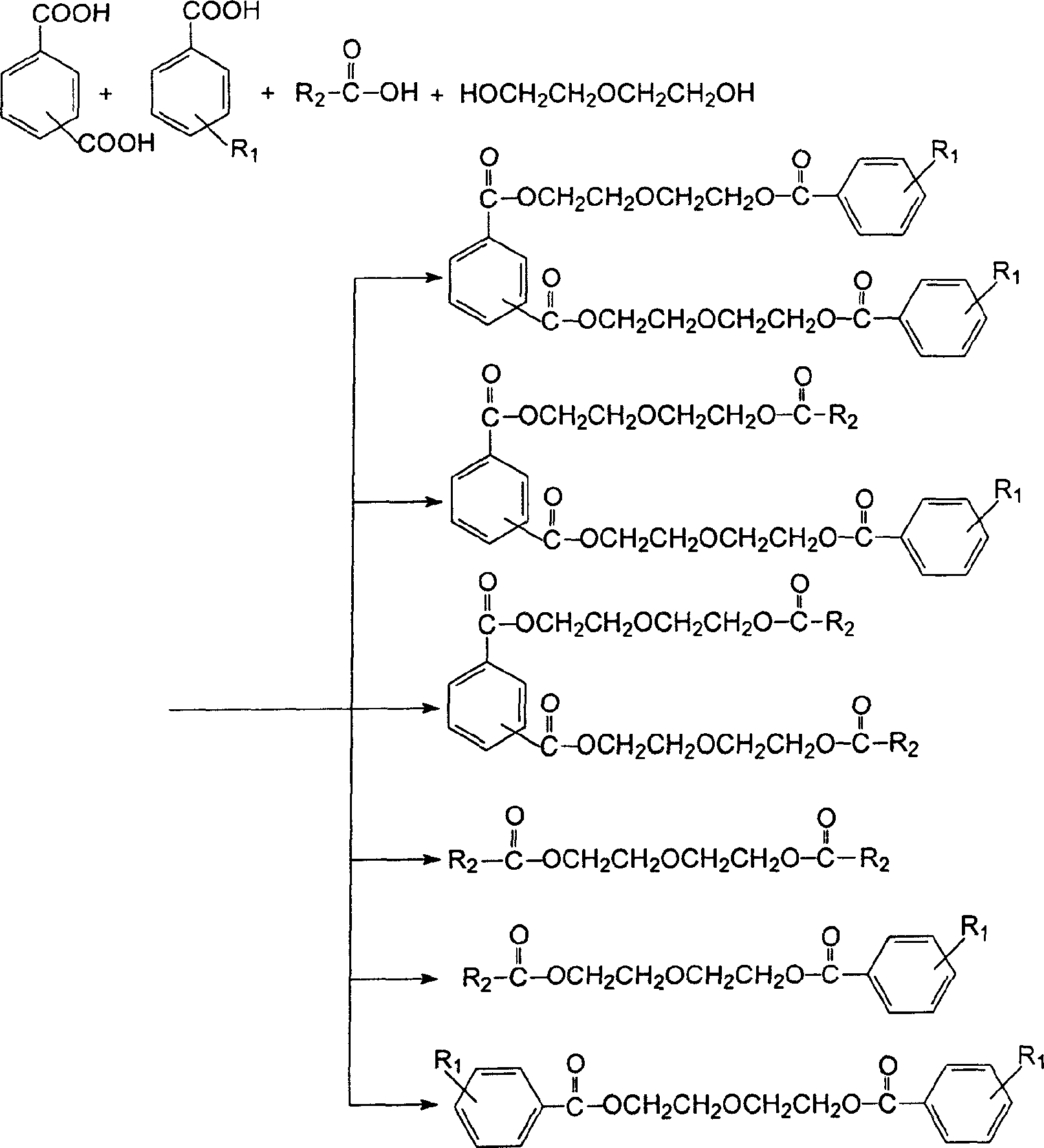
[0028] Put the measured CTA residue, formic acid, ethylene glycol and sulfuric acid into an esterification tank with a water separation system, and heat it to boiling under normal pressure. The water in the CTA residue and the water generated by esterification are continuously discharged from the water separator separated out. After most of the water is azeotropically distilled off, continue heating until no water is distilled off. Stay at this temperature for a period of time and measure its acid value. When the acid value is close to the acid value of the acid catalyst added, it is regarded as the end point of esterification, and the temperature is lowered to about 80°C, and then enters the neutralization stage.
[0029]Add an aqueous sodium carbonate solution whose consumption is 1.5 times the acid value measured to the above mixed crude ester, stir for half an hour, then settle still, and separate the water layer to obtain the neutralized crude ester. Heat the crude este...
Embodiment 2-7
[0031] The procedure of Example 1 was repeated except that different starting materials and reaction conditions were used. The specific raw materials, reaction conditions and results of each embodiment are listed in Table 1.
[0032] Example 1
Example 2
Example 3
Example 4
Example 5
Example 6
Example 7
fatty acid
C 3-7 Monobasic acid
C 3-7 Monobasic acid
C 8-10 Monobasic acid
Ethylene glycol
Decanediol
CTA Residue: Fatty Acid:
The molar ratio of glycol
1.0∶0.8∶1.0
1.0∶9.0∶5.0
1.0∶0.4∶0.8
1.0∶0.2∶0.9
1.0∶4.5∶3.0
1.0∶0.7∶0.9
5
1.0∶0.3∶0.8
5
catalyst
Toluenesulfonic acid
Potassiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com