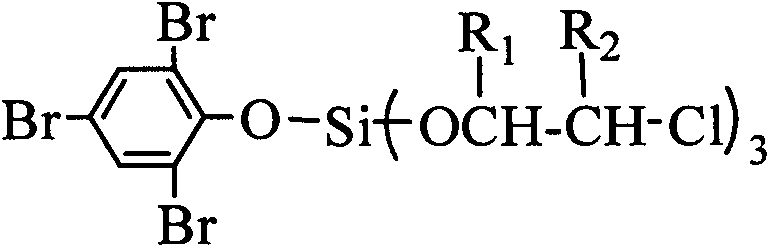A kind of flame retardant tribromophenoxy tris (dichloropropoxy) silane compound and preparation method thereof
A technology of tribromophenoxytri-dichloropropoxy, which is applied in the direction of silicon organic compounds, can solve problems such as difficulty in finding substitutes, achieve easy purification and separation, overcome the effect of easy volatility of the reaction, and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
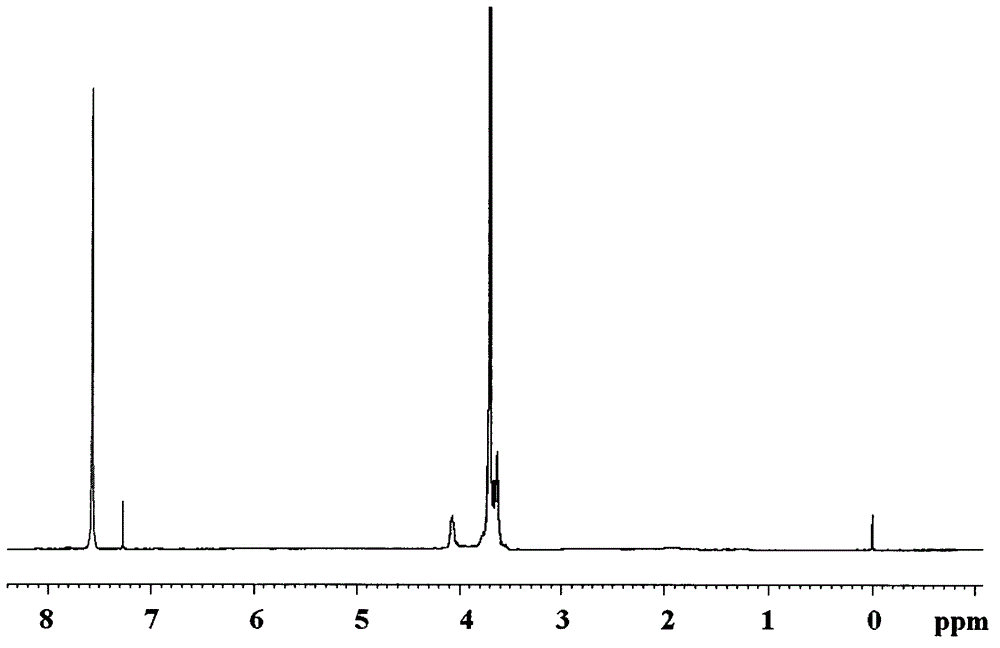
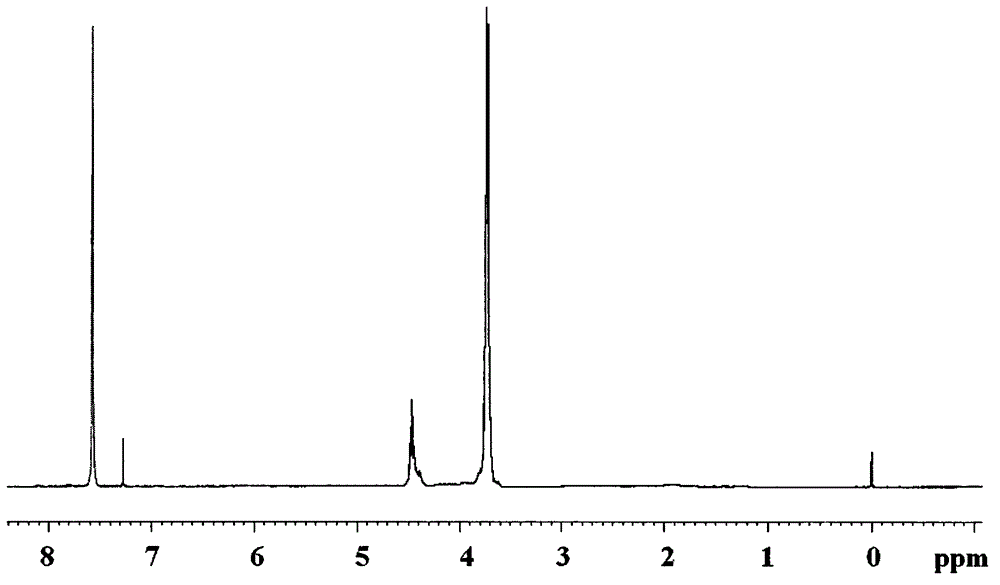
[0031] Example 1 In the 250ml four-neck flask equipped with agitator, thermometer, dropping funnel and high-efficiency reflux condenser and a hydrogen chloride absorbing device connected in series with a drying tube at the top of the condenser, replace the air in the bottle with nitrogen , add 80ml of dioxane and 16.99g (0.1mol) of silicon tetrachloride, under stirring, cool with an ice-water bath, reduce the temperature of the reaction system to 0°C, and drop 12.90g of silicon tetrachloride into the organic solution (0.1mol) 2,3-dichloro-1-propanol, the temperature of the dropwise addition process is controlled below 8°C, after the drop is completed, the temperature is raised to 35°C in 1h, and the heat preservation reaction is carried out for 1h. After the hydrogen chloride is released, add 33.08g ( 0.1mol) tribromophenol, heat up to 80°C, keep warm for 2 hours, after the hydrogen chloride is released, cool down to below 25°C, then add 25.80g (0.2mol) 2,3-dichloro-1-propanol ...
Embodiment 2
[0032]Example 2 In a 250ml four-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a high-efficiency reflux condenser and a hydrogen chloride absorption device connected in series with a drying tube at the top of the condenser, replace the air in the bottle with nitrogen , add 80ml of ethylene glycol dimethyl ether and 16.99g (0.1mol) of silicon tetrachloride, under stirring, cool with an ice-water bath, reduce the temperature of the reaction system to 0°C, and add dropwise to the organic solution of silicon tetrachloride 12.90g (0.1mol) 2,3-dichloro-1-propanol, the dropwise addition process controls the temperature below 8°C, after the drop is completed, the temperature is raised to 35°C for 1h, and the temperature is kept for 1h. After the hydrogen chloride is released, add 33.08 g (0.1mol) tribromophenol, heat up to 60°C, keep warm for 4 hours, after the hydrogen chloride is released, cool down to below 25°C, then add 25.80g (0.2mol) 2,3-dichloro-1-pr...
Embodiment 3
[0033] Example 3 In a 250ml four-necked flask equipped with a stirrer, a thermometer, a dropping funnel and a high-efficiency reflux condenser and a hydrogen chloride absorbing device connected in series with a drying tube at the top of the condenser, replace the air in the bottle with nitrogen , add 80ml acetonitrile and 16.99g (0.1mol) silicon tetrachloride, under stirring, cool with ice-water bath, make reaction system temperature drop to 0 ℃, in the organic solution of silicon tetrachloride, add dropwise 12.90g (0.1mol) ) 2,3-dichloro-1-propanol, the dropwise addition process controls the temperature below 8°C, after the drop is completed, the temperature is raised to 35°C for 1h, and the temperature is kept for 1h, after the hydrogen chloride is released, add 33.08g (0.1mol) Tribromophenol, heat up to 70°C, keep warm for 3 hours, after the hydrogen chloride is released, cool down to below 25°C, then add 25.80g (0.2mol) 2,3-dichloro-1-propanol dropwise, and control the temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com