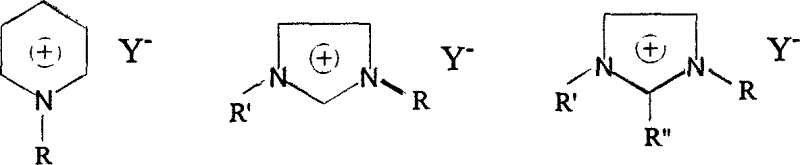
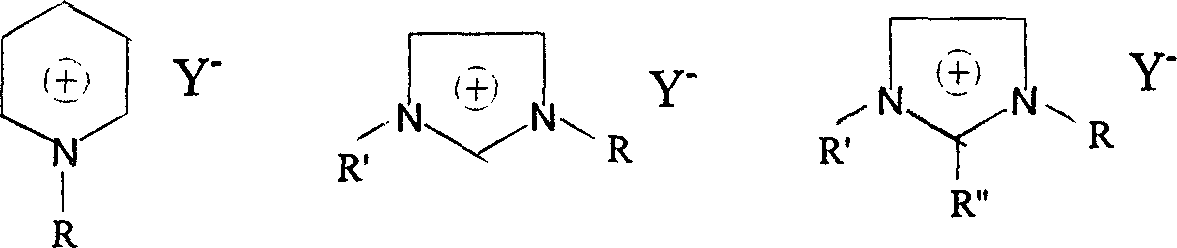
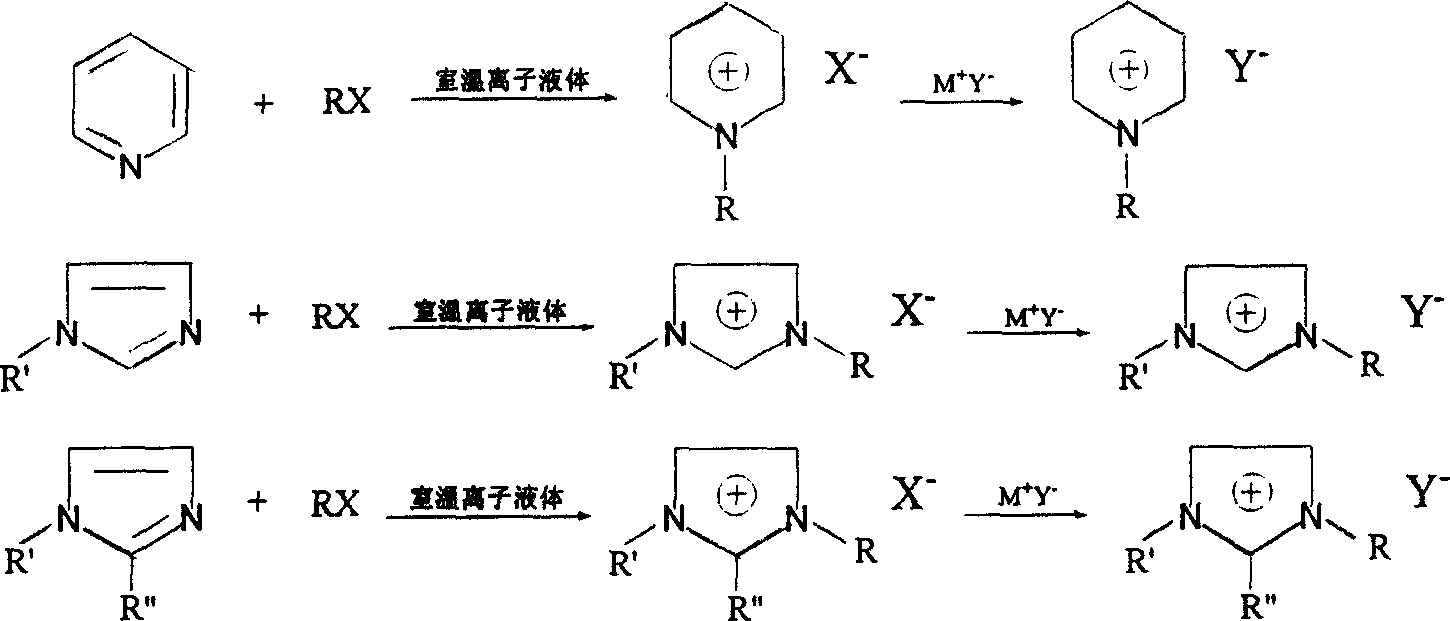
Environmental protection Method for synthesizing room temperature ion liquid
A technology of room temperature ionic liquid and synthesis method, which is applied in the field of green synthesis of room temperature ionic liquid, can solve the problems of unsuitable large-scale synthesis of room temperature ionic liquid, difficult control of reaction conditions by microwave method, and a large amount of organic solvents, and achieves environmental friendliness and product quality. Good, high reaction yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 100mL three-necked flask, add 18.4g potassium hexafluorophosphate, 19.3g 1-bromooctane, 8.2g N-methylimidazole and 20.0g 1-n-octyl-3-methylimidazolium hexafluorophosphate ion Liquid, stirred at 135°C for 5 hours. After the reaction was completed, 25 mL of water was added, stirred thoroughly, and left to separate the water phase. The ionic liquid phase was washed with 20 mL×3 water for 3 times, and then the residual water was removed under reduced pressure to obtain 48.4 g of 1-n-octyl-3- Methyl hexafluorophosphate ionic liquid, 28.4g of ionic liquid was newly generated in the actual reaction, and the yield was 83.5%.
Embodiment 2
[0021] In a 50mL three-necked flask, sequentially add 11.0g sodium fluoroborate, 14.0g 1-chlorobutane, 9.6g 1,2-dimethylimidazole and 20.0g 1-n-butyl-2,3-dimethylimidazole Tetrafluoroborate ionic liquid, stirred and reacted at 80°C for 35 hours. After the reaction, transfer the reaction solution to a 150mL flask, add 40mL of water, stir thoroughly, then extract the ionic liquid in the water phase with 30mL×5 dichloromethane for 5 times, combine the dichloromethane extracts, and distill to remove the solvent , 39.5g of 1-n-butyl-2,3-dimethylimidazolium tetrafluoroborate ionic liquid was obtained, and 19.5g of ionic liquid was newly generated by the actual reaction, with a yield of 81.3%.
Embodiment 3
[0023] In a 100mL three-necked flask, add 9.4g lithium fluoroborate, 17.9g 2-bromoheptane, 8.2g N-methylimidazole and 25.0g 1-(1-methylhexyl)-3-methylimidazolium tetrafluoro Borate ionic liquid, stirred and reacted at 100°C for 12 hours. After the reaction was completed, 25 mL of water was added, stirred thoroughly and allowed to stand to separate the water phase. The ionic liquid phase was washed with 20 mL×3 water for 3 times, and then the residual water was removed under reduced pressure to obtain 48.3 g of 1-(1-methylhexyl) - 3-methylimidazolium tetrafluoroborate ionic liquid, 23.3 g of ionic liquid was newly generated in the actual reaction, and the yield was 86.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com