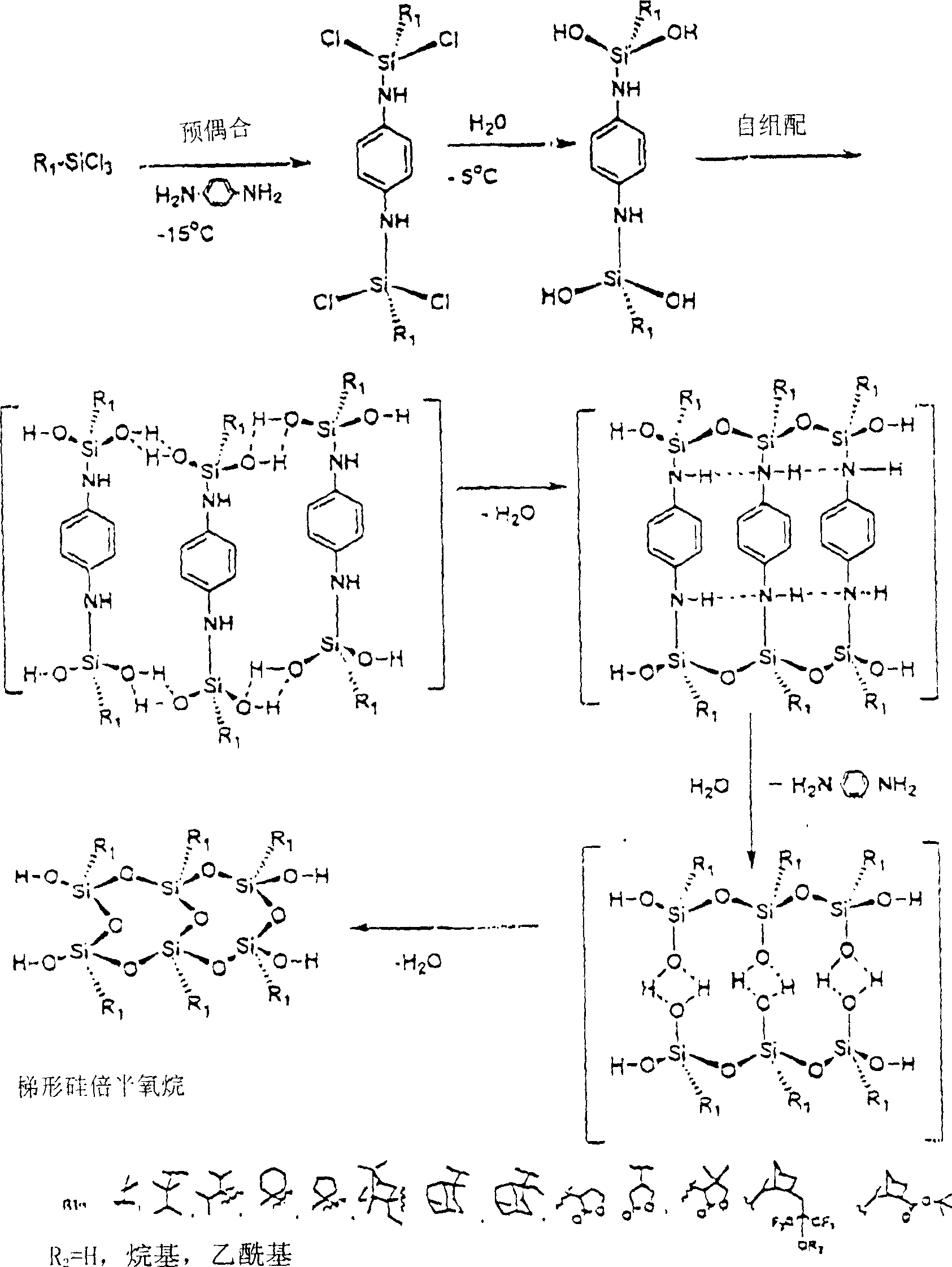
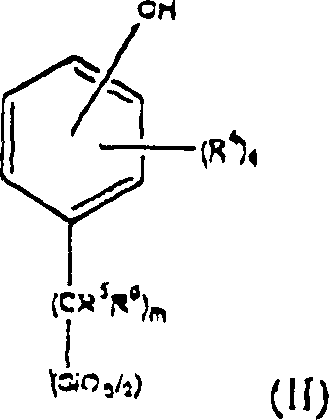
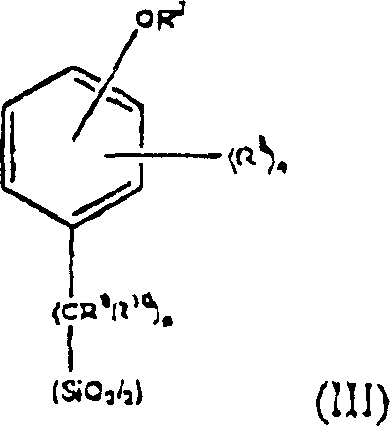
Polyorganosiloxane and preparing method for photoresist composition containing the said polyorganosiloxane
A photoresist and silane compound technology, which is applied in the field of diamine reagents, can solve the problems of increased resist thickness variation and reduced quality of electronic components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1: the synthesis of polymer
[0112] A solution of known content of 1,4-phenylenediamine, triethylamine and excess THF was added dropwise to a three-necked flask containing -15 known content of alkyl-substituted trichlorosilane. The solution was stirred at low temperature (-15) for 30 minutes, then known amounts of water and triethylamine and THF were added dropwise at -5. The mixture was stirred for a further 3 hours at this temperature, then washed to neutral with water and dried over anhydrous sodium sulfate overnight.
[0113] The final solution from the above reaction was stirred at 50° C. for 72 hours in the presence of molecular sieves (4 Angstroms) and a catalytic amount of triethylamine. After 72 hours, the polymer solution was washed neutral with water and the solvent was distilled off. The solid polymer was dissolved in a minimum amount of THF and precipitated in water (twice) and dried under vacuum at 50 for 24 hours.
Embodiment 2
[0114] Example 2: Photoresist Preparation and Lithographic Processing
[0115] A preferred bilayer resist composition is prepared and processed as follows
[0116] top floor
[0117] The top resist was formulated at 10% by weight solids. The following components are mixed to provide a resist composition: polymer, base additive, surfactant, and photoacid generator components.
[0118] The polymer, base additive (Troger's base) and surfactant (RO-8 surfactant) were added as a solution in propylene glycol methyl ether acetate (PGMEA). A solution of photoacid generator in ethyl lactate was added. The final solvent blend of the formulated resist was 90:10 v / v PGMEA:ethyl lactate. The polymer was prepared as in Example 1 above. The photoacid generator component consisted of 6.5% by weight of MDT on total solids (all resist components except solvent) and 2.9% by weight of tert-butylphenyldiphenyltrifluorophenylsulfonate based on total solids Matte composition. The base addit...
Embodiment 3
[0123] Embodiment 3: the synthesis of polymer
[0124] Part 1: Acetylation of NB-HF-OH(A)
[0125] Step 1: Acetylation of NB-HFOH
[0126]
[0127]NB-HFOH(A) alkenes were acetylated using anhydrides in the presence of dimethylaminopyridine (DMAP) in tetrahydrofuran (THF) at room temperature. Olefin conversion was monitored by gas chromatography (GC). The reaction became complete (>99%) within 4 hours. After the reaction, THF was distilled off, and the crude viscous oil was dissolved in dichloromethane and extracted with sodium bicarbonate solution, followed by washing with water until the solution became neutral. The dichloromethane layer was separated and evaporated in vacuo to give the product NB-HFOAc (B) as a clear viscous oil. use 1 H. 13 C. 19 FNMR Characterization B.
[0128] Part 2. Hydrosilylation of NB-HFOAcB:
[0129] Step 2: Hydrosilylation of NB-HFOAc
[0130]
[0131] Karstedt's catalyst (Pt 0 ), with HSiCl 3 Compound B was hydrosilylated for 36...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com