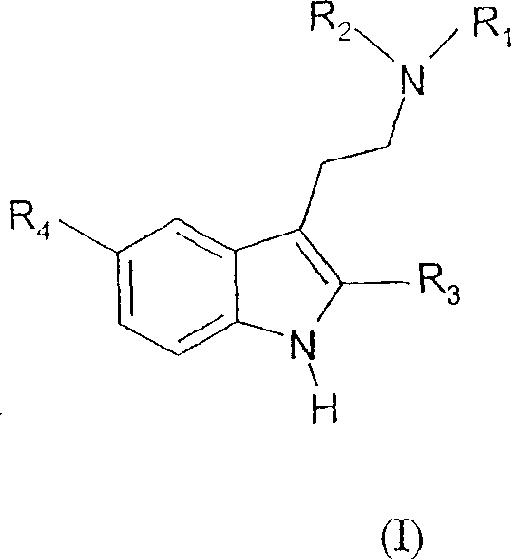
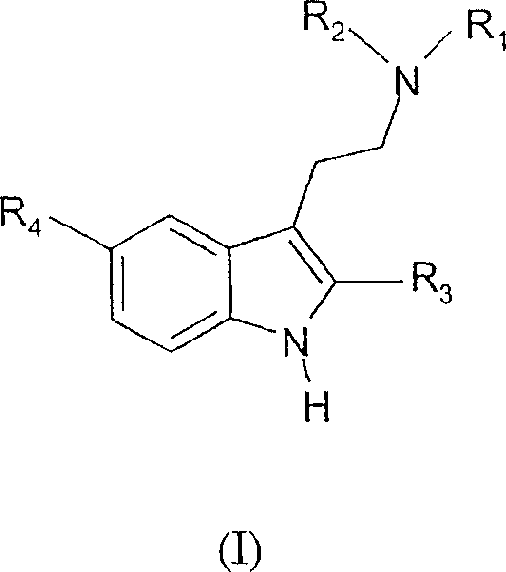
5-halo-tryptamine derivatives used as ligands of 5-HT 6 and/or 5-HT7 serotonin receptors
A 5-HT75-, 5-HT6 technology, applied in the field of 5-halotryptamine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
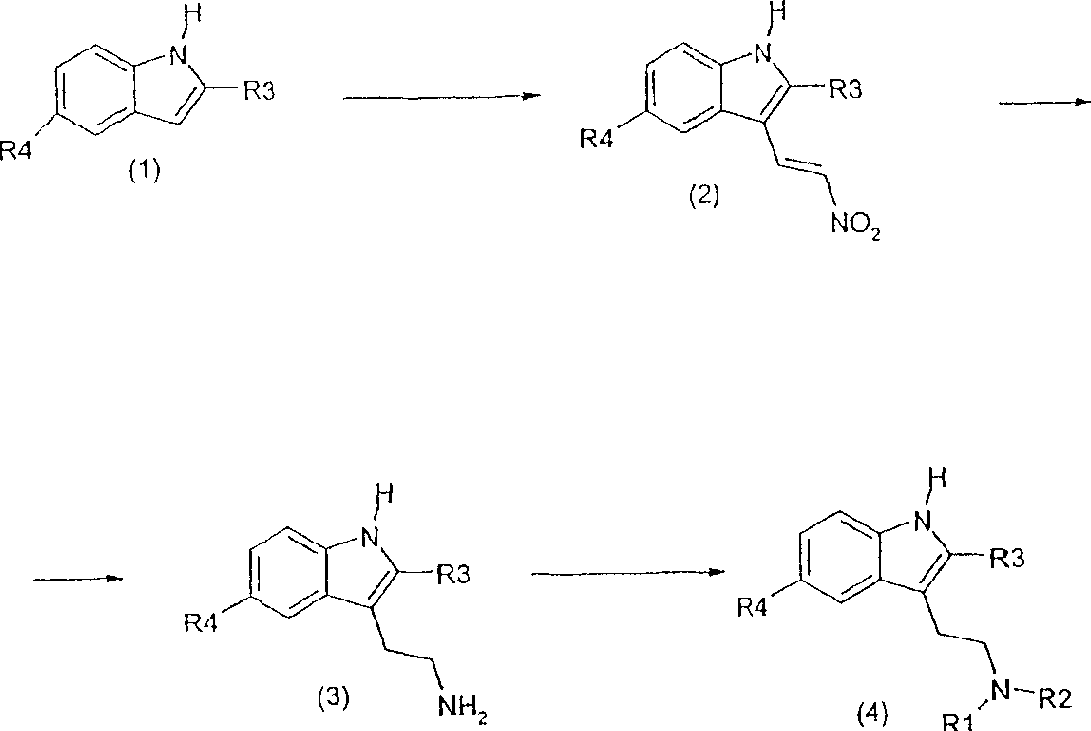
Embodiment 1
[0047] (E)-5-Bromo-2-methyl-3-(2-nitrovinyl)-1H-indole
[0048] A solution of 0.58 g of 1-(dimethylamino)-2-nitroethylene (5 mmol) in 5 mL of trifluoroacetic acid was stirred and cooled to 0 °C, and 1.05 g (5 mmol) of 5-bromo-2-methyl -indole, and react the resulting mixture at room temperature under a nitrogen atmosphere for 30 minutes. The reaction mixture was then placed in an ice-water bath. The aqueous solution was extracted with ethyl acetate, and the combined organic phases were then washed with saturated bicarbonate solution, followed by water, and finally dried over anhydrous sodium sulfate. After filtration, the solvent was removed under reduced pressure to leave a solid, orange residue which was then suspended in ethyl acetate-ether mixture and filtered.
[0049] Yield: 89%
[0050] R f =0.3 (cyclohexane / EtOAc:1)
[0051] M.p.: 196-198°C (decomposition)
[0052] 1H-NMR (200MHz) (DMSO-d 6 ): δ2.59(s, 3H), 7.34(m, 2H), 7.97(d, 1H, J=13.2Hz), 8.06(m, 1H), 8.26(...
Embodiment 2
[0067] According to the described method, and according to the above scheme and examples, the following compounds were prepared:
[0068] (E)-5-Chloro-2-methyl-3-(2-nitrovinyl)-1H-indole
[0069] orange solid
[0070] Yield: 85%;
[0071] M.p..191-193℃
[0072] 1 H NMR (200MHz, (acetone-d 6 ): δ2,68(s,3H), 7,21(dd,1H, J=1,95 and J=8,5Hz), 7,5(d,1H, J=8,5Hz), 7,85 (d, 1H, J=13,3Hz), 7,86 (d, 1H, J=1,95Hz), 8,30 (d, 1H, J=13,3Hz); EIMS: m / z 236 (M + ), 154(100).
[0073] 5-Chloro-2-methyltryptamine hydrochloride
[0074] From EtOH / Et 2 O Precipitated crystalline pale brown solid.
[0075] Yield: 72%
[0076] 1 H NMR (200MHz, (DMSO-d 6 ): δ2,33(s,3H), 6,97(dd,1H, J=1,9 and J=8,3Hz), 7,25(d,1H, J=8,3Hz), 7,52 (d, 1H, J=1,5Hz), 8,03(br,s,3H), 11,15(s,1H).
[0077] 5-chloro-2-methyl-N,N-dimethyltryptamine (ST 1936)
[0078] white solid;
[0079] Yield: 75%;
[0080] M.p.=126-127℃
[0081] 1 H NMR (200MHz, CDCl 3 ): δ2,35(s,6H), 2,38(s,3H), 2,44-2,52(m,2H), 2,79-2...
Embodiment 3
[0084]
[0085] Reactants: (a) t-BuLi, THF, -20°C; EtI, -78° to room temperature, 2h; (b) 2N NaOH, MEOH, reflux, 40h; (c) 1-(dimethylamino)- 2 Nitroethene, TFA, 0°C, 0,5h; (d) LiAlH 4 , THF, room temperature, 6h; (e) NaCNBH 3 , 40%, HCHO, MeOH, AcOH, room temperature, 2,5h.
[0086] N-(phenylsulfonyl)-5-chloro-2-ethylindole (2).
[0087] t-BuLi (3.7 mL of a 1.7 M solution in pentane) was added dropwise to N-(phenylsulfonyl)-5-chloroindole (1) in THF (35 mL) at -70 °C under nitrogen atmosphere (J. Org. Chem. (Journal of Organic Chemistry) 1981, 46, 3859) (1.5 g, 5.14 mmol). The mixture was stirred for 15 minutes, warmed to room temperature over 20 minutes, cooled to -70 °C, and treated with iodoethane solution (0.84 mL, 10.5 mmol) in anhydrous THF (5 mL). The mixture was stirred at -78 °C for 1 h, allowed to warm to room temperature, stirred for 2 h, poured into ice (15 g) and saturated NH 4 Cl aqueous solution, then extracted with diethyl ether (3 x 20 mL). The combin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com