Namenda synthesis method
A technology of memantine hydrochloride and its synthetic method, which is applied in the field of synthesis of memantine hydrochloride, can solve the problems of chloroform harmful to human body, low boiling point of ether, volatilization of ether, etc., and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
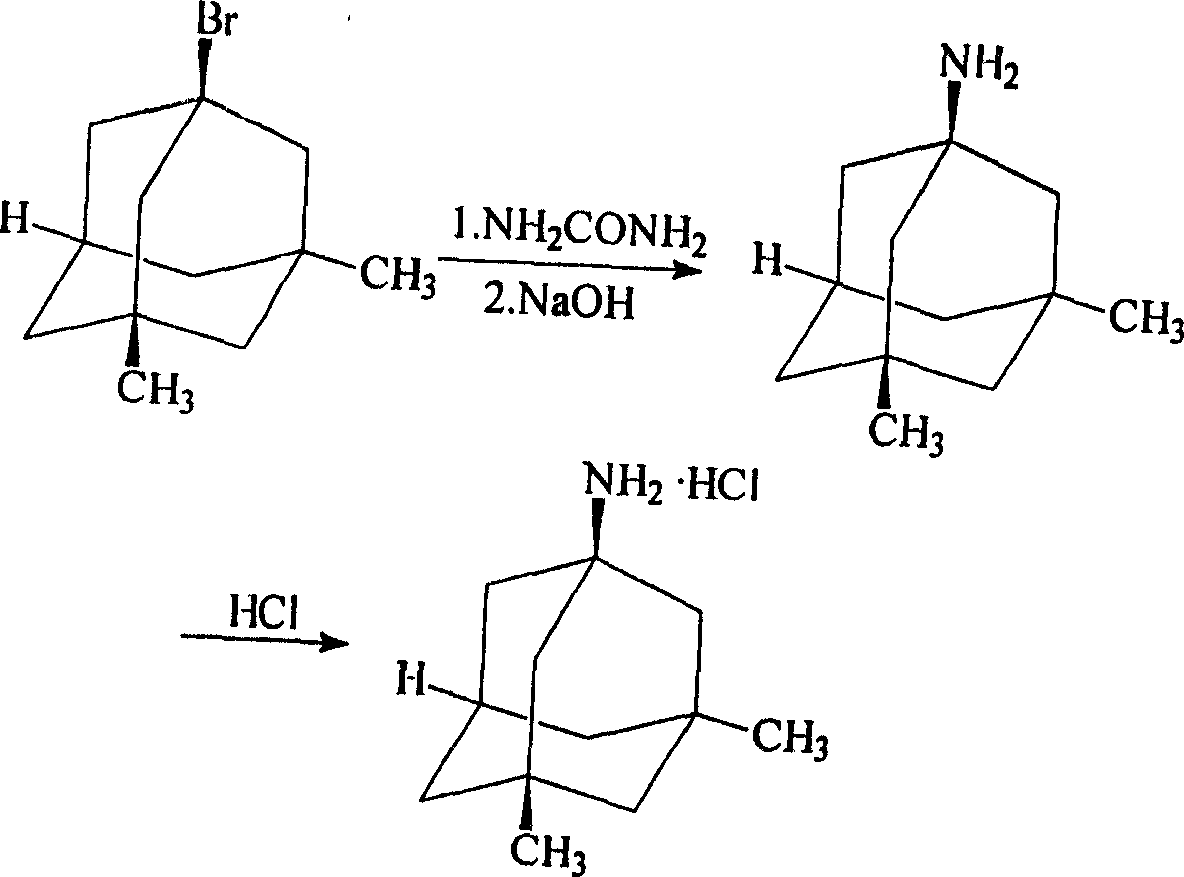
[0039] Weigh 100g of bromodimethyladamantane and 20g of urea respectively, mix the bromodimethyladamantane and urea evenly, heat and melt until the temperature is 180°C, and the internal temperature reaches 180-240°C; after 4 hours of reaction, cool down naturally , add concentrated hydrochloric acid to dissolve the reactants completely; transfer to a beaker, add NaOH to adjust the pH to 7.0, steam distillation; filter and dry to obtain dimethylamantadine.
[0040] Add dimethylamantadine, distilled water, and activated carbon into the flask; adjust the pH to 1.1 with hydrochloric acid, heat to 70°C to dissolve and decolorize for 0.5 hours, and cool to room temperature; filter, and evaporate the filtrate to dryness under reduced pressure to obtain a white solid, which is washed with acetone until The pH was 5.0, and the filter was dried; the filter cake was vacuum-dried at 75° C. to obtain memantine hydrochloride with a total yield of 56.2%.
Embodiment 2
[0042] Weigh 100g of bromodimethyladamantane and 200g of urea respectively, mix the bromodimethyladamantane and urea evenly, heat and melt until the temperature is 160°C, and the internal temperature reaches 180-240°C; after reacting for 4.5 hours, cool down naturally , add concentrated hydrochloric acid to completely dissolve the reactants; transfer to a beaker, add NaOH to adjust the pH to 8.0, steam distillation; filter and dry to obtain dimethylamantadine.
[0043] Add dimethylamantadine, distilled water, and activated carbon into the flask; adjust the pH to 3.0 with hydrochloric acid, heat to 72°C to dissolve and decolorize for 1 hour, and cool to room temperature; filter, and evaporate the filtrate to dryness under reduced pressure to obtain a white solid, which is washed with acetone until The pH was 4.0, filtered and dried; the filter cake was dried in an oven at 75° C. to obtain memantine hydrochloride with a total yield of 52.5%.
Embodiment 3
[0045] Weigh 100g of bromodimethyladamantane and 100g of urea respectively, mix bromodimethyladamantane and urea evenly, heat and melt until the temperature is 140°C, and the internal temperature reaches 180-240°C; cool down naturally after 4 hours of reaction , add concentrated hydrochloric acid to dissolve the reactants completely; transfer to a beaker, add NaOH to adjust the pH to 13.9, steam distillation; filter and dry to obtain dimethylamantadine.
[0046] Add dimethylamantadine, distilled water, and activated carbon into the flask; adjust the pH to 6.0 with hydrochloric acid, heat to 71°C to dissolve and decolorize for 1 hour, and cool to room temperature; filter, and evaporate the filtrate to dryness under reduced pressure to obtain a white solid, which is washed with ethanol until The pH was 6.0, and the filter was dried; the filter cake was vacuum-dried at 65° C. to obtain memantine hydrochloride with a total yield of 51.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com