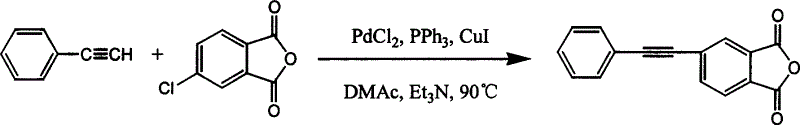4-phenylacetylene benzoic anhydride preparation method
A technology of phenylacetylene phthalic anhydride and phenylacetylene, which is applied in the field of preparation of 4-phenylacetylene phthalic anhydride, can solve the problems of restricting the development of PI resin and the high price of the final product, and achieve the effects of low price, simple preparation method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
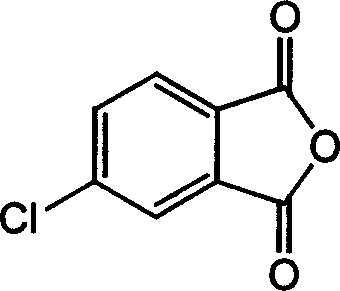
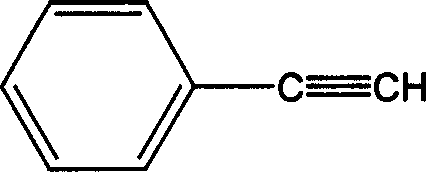
[0018] Under nitrogen protection, 4-chlorophthalic anhydride (182.56g, 1mol), phenylacetylene (102.13g, 1mol), palladium dichloride (0.018g 0.1mmol), triphenylphosphine (0.1g 0.4mmol), chlorine Cuprous chloride (0.02g 0.2mmol) was dissolved in N,N'-dimethylacetamide (1800ml) and triethylamine (1000ml), heated to 80°C, after the reaction was carried out for 6h, the filtrate was poured into a large amount of water, The precipitate was collected and fully washed with water. After drying, it was heated and vacuum sublimated to obtain pale yellow 4-phenylacetylene phthalic anhydride (174 g 0.7 mol) with a yield of 70%.
Embodiment 2
[0020] Under nitrogen protection, 4-chlorophthalic anhydride (182.56g, 1mol), phenylacetylene (102.13g, 1mol), palladium dichloride (0.07g, 0.4mmol), triphenylphosphine (0.06g, 0.24mmol) 1. Cuprous chloride (0.6g, 0.6mmol) was dissolved in N,N'-dimethylformamide (1200ml) and triethylamine (500ml), heated to 60°C, after the reaction was carried out for 20h, the filtrate was poured into The precipitate was collected and washed in a large amount of water, and after being fully dried, it was sublimated under vacuum by heating to obtain 4-phenylacetylene phthalic anhydride (149 g, 0.6 mol) with a yield of 60%.
Embodiment 3
[0022] Under nitrogen protection, 4-chlorophthalic anhydride (182.56g, 1mol), phenylacetylene (102.13g, 1mol), palladium dichloride (0.18g, 1mmol), triphenylphosphine (1g, 4mmol), chloride Cuprous (0.1g, 10mmol) was dissolved in N-methyl-2-pyrrolidone (600ml) and triethylamine (500ml), heated to 80°C, and after 12 hours of reaction, the filtrate was poured into a large amount of water, and the precipitate was collected And washed, fully dried, heated and vacuum sublimated to obtain pale yellow 4-phenylacetylene phthalic anhydride (179 g, 0.72 mol) with a yield of 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com